��Ŀ����
����Ŀ������ѧһѡ��3:���ʽṹ�����ʣ�1735����仯ѧ�Ҳ�����(G��Brands)�Ƴ������ܡ��ܵĿ�����ܵĻ�����һֱ�����մɡ����������ŵ����ϡ���20���ͣ��ܼ���Ͻ��ڵ������е�����������պͺ���ȹ�ҵ���ŵõ��㷺��Ӧ�ã�����Ϊһ����Ҫ��ս�Խ����������ܼ��仯���������Ҫ���ã��ش���������:
(1)��̬Coԭ�ӵĵ����Ų�ʽΪ___________��
(2)[Co(NH3)5H2O]Cl3��һ��ש��ɫ�ľ��壬��ͨ��CoCl2��NH4Cl��Ũ��ˮ��H2O2�Ƶá�
��Co��N��0ԭ�ӵĵ�һ�������ɴ�С��˳����__________��
��[Co(NH3)5H2O]Cl3��CoԪ�ػ��ϼ�Ϊ____������λԭ��Ϊ_____��1mol�þ����к���____mol�Ҽ���
��H2O2��Oԭ�ӵ��ӻ����������______��H2O2����ˮ���ܣ������Ǽ��Է����⣬����Ϊ____��
��NH3��NF3�Ŀռ乹�Ͷ���ͬ,��Co3+����NH3�γ������ӣ���NF3���ܡ�ԭ����________��
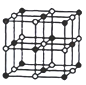
(3)CoO������ͼ����֪Coԭ�Ӱ뾶Ϊapm,Oԭ�Ӱ뾶Ϊbpm����Ⱦ����������Oԭ��Χ�ɵĿռ���״Ϊ_____���þ�����ԭ�ӵĿռ�������Ϊ__________(�ú�a��b�ļ���ʽ��ʾ)��
���𰸡� 1s22s22p63s23p63d74s2����[Ar]3d74s2�� N>O>Co +3 N ��O 23 sp3 H2O2��ˮ���Ӽ����γ���� N��F��H����Ԫ�صĵ縺��F>N>H����NF3�У����õ��Ӷ�ƫ���ԭ�ӣ�ƫ�뵪ԭ�ӣ�ʹ�õ�ԭ���ϵŶԵ���������Co3+�γ���λ�� �������� ![]()
��������(1) CoΪ27��Ԫ�أ�ԭ�Ӻ��������Ϊ27�������������ԭ����д��������Ų�ʽ��
��2����ͬһ����Ԫ�صĵ�һ����������ԭ������������������������������IIA��͵�VA��Ԫ�صĵ�һ�����ܴ�������Ԫ����
��[Co(NH3)5H2O]Cl3��Co3+���������ӣ�NH3��H2OΪ��λ�壬�Դ˷�����
��H2O2����H2O2�Ľṹ����֪����Oԭ���γ�1��O-H����1��O-O��������2�Թ¶Ե����ӻ������Ϊ4���ӻ���ʽΪsp3��H2O2������ˮ������ΪH2O2������H2O���Ӽ���γ������
��N��F��H����Ԫ�صĵ縺�ԣ�F��N��H������NH3�й��õ��Ӷ�ƫ��N������NF3�У����õ��Ӷ�ƫ��F��ƫ��Nԭ����
(3)���ݾ����Ľṹͼ����֪���������к���Coԭ����Ϊ4����ԭ����Ϊ4����ԭ��λ������е�����ģ���Ⱦ����������Oԭ��Χ�ɵĿռ���״Ϊ�������壻�ܡ��������Ӱ뾶�ֱ�Ϊanm��bnm,����������ԭ�ӵ����Ϊ=4��![]() ��anm��3+4��
��anm��3+4��![]() ��anm��3�����ݾ����Ľṹͼ����֪���������ı߳�Ϊ(2anm+2bnm)���������Ϊ(2anm+2bnm)3���ݴ˼�����
��anm��3�����ݾ����Ľṹͼ����֪���������ı߳�Ϊ(2anm+2bnm)���������Ϊ(2anm+2bnm)3���ݴ˼�����
���:(1) CoΪ27��Ԫ�أ�ԭ�Ӻ��������Ϊ27�������������ԭ�������������Ų�ʽΪ��1s22s22p63s23p63d74s2��[Ar]3d74s2��
�ʴ�Ϊ��1s22s22p63s23p63d74s2��[Ar]3d74s2��
��2����N��O����ͬһ����Ԫ����ԭ������N<O��ͬһ����Ԫ�صĵ�һ����������ԭ����������������������ڢ�A��Ĵ��ڵڢ�A�������Co�ǽ�������һ��������С���������һ�����ܴ�С˳����N>O>Co,��ˣ�������ȷ������N>O>Co��
��[Co(NH3)5H2O]Cl3��Co3+���������ӣ�NH3��H2OΪ��λ�壬�ɴ˿�֪CoԪ�ػ��ϼ�Ϊ+3�ۣ�����λԭ��ΪN ��O ��1mol�þ����к���3��5molN-H����2molH-O����6mol��λ��������23mol�Ҽ���
��ˣ�������ȷ����: +3 ��N ��O ��23 ��
��H2O2����H2O2�Ľṹ����֪����Oԭ���γ�1��O-H����1��O-O��������2�Թ¶Ե����ӻ������Ϊ4���ӻ���ʽΪsp3��H2O2������ˮ������ΪH2O2������H2O���Ӽ���γ������
��ˣ�������ȷ������sp3 ��H2O2��ˮ���Ӽ����γ������
��N��F��H����Ԫ�صĵ縺�ԣ�F��N��H������NH3�й��õ��Ӷ�ƫ��N������NF3�У����õ��Ӷ�ƫ��F��ƫ��Nԭ�ӣ��ʴ�Ϊ��F�ĵ縺�Ա�N��N-F�ɼ����Ӷ���Fƫ�ƣ�����NF3��Nԭ�Ӻ˶���¶Ե��ӵ�����������ǿ�������γ���λ������NF3������Co3+�γ������ӣ�����N��F��H����Ԫ�صĵ縺�ԣ�F��N��H����NF3�У����õ��Ӷ�ƫ��F��ƫ��Nԭ��ʹ�õ�ԭ���ϵŶԵ���������Co3+�γ���λ��������
��ˣ�������ȷ������N��F��H����Ԫ�صĵ縺��F>N>H����NF3�У����õ��Ӷ�ƫ���ԭ�ӣ�ƫ�뵪ԭ�ӣ�ʹ�õ�ԭ���ϵŶԵ���������Co3+�γ���λ����
��3�����ݾ����Ľṹͼ����֪��,�����к���Coԭ����Ϊ4����ԭ����Ϊ4����ԭ��λ������е�����ģ���Ⱦ����������Oԭ��Χ�ɵĿռ���״Ϊ�������壻�ܡ��������Ӱ뾶�ֱ�Ϊanm��bnm,����������ԭ�ӵ����Ϊ=4��![]() ��anm��3+4��
��anm��3+4��![]() ��anm��3�����ݾ����Ľṹͼ����֪���������ı߳�Ϊ(2anm+2bnm)���������Ϊ(2anm+2bnm)3��
��anm��3�����ݾ����Ľṹͼ����֪���������ı߳�Ϊ(2anm+2bnm)���������Ϊ(2anm+2bnm)3��
�þ����Ŀռ�������Ϊ��
![]() ��100%=
��100%=![]() ��100%
��100%
��ˣ�������ȷ���������������� ![]() ��100%��
��100%��

 Сѧ�̲�ȫ��ϵ�д�
Сѧ�̲�ȫ��ϵ�д� Сѧ��ѧ������ѿڶ���ϵ�д�
Сѧ��ѧ������ѿڶ���ϵ�д� ������Ӧ�������������ϵ�д�
������Ӧ�������������ϵ�д� �㽭֮�ǿ�ʱ�Ż���ҵϵ�д�
�㽭֮�ǿ�ʱ�Ż���ҵϵ�д�����Ŀ����֪��2SO2 (g)+ O2(g) ![]() 2SO3(g) ��H���й��������£�
2SO3(g) ��H���й��������£�
T������ | 527 | 627 | 727 | 827 | 927 |
ƽ�ⳣ��K | 910 | 42 | 3.2 | 0.39 | 0.12 |
����˵������ȷ����
A. ����ƽ�ⳣ�����¶ȵı仯��ϵ���жϳ���H��0
B. ���������������䣬SO2��ƽ��ת������(727��)����(927��)
C. ����ѹǿ�������¶������SO2��ת����
D. SO3���ȶ������¶ȵ����߶�����