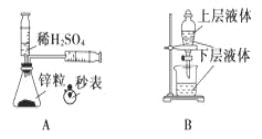
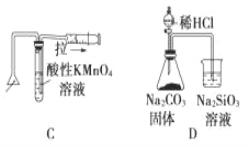
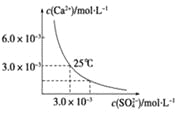
��Ŀ����
����Ŀ���й����ʵ�ת����ϵ����ͼ��ʾ(������������������ȥ)��A��C��E��G����ѧ��ѧ�г����ĵ��ʣ�ͨ��״���£�E���ܶ���С�����壬G�ǻ���ɫ�����壬B�dz�������ɫҺ�壬F��ǿ������ɫ��Ӧ����ʻ�ɫ��D��ij�־��д��ԵĽ����������Ҫ�ɷ֣�K���ȷֽ�����ɺ���ɫ���塣
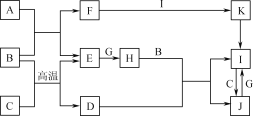
��ش��������⣺
(1) D�Ļ�ѧʽΪ____________��
(2) F�ĵ���ʽΪ____________��
(3) д��J��Һ�е���ϡ���ᷴӦ�����ӷ���ʽ��________________________��
(4) д��������G��F����Һ��Ӧ�Ļ�ѧ����ʽ��________________________��
���𰸡�Fe3O4 ![]() 3Fe2+��4H+��NO3-===3Fe3+��NO��+2H2O Cl2��2NaOH===NaCl��NaClO��H2O
3Fe2+��4H+��NO3-===3Fe3+��NO��+2H2O Cl2��2NaOH===NaCl��NaClO��H2O
��������
A��C��E��G����ѧ��ѧ�г����ĵ��ʣ�ͨ��״���£�E���ܶ���С�����壬Ϊ������G�ǻ���ɫ�����壬Ϊ������B�dz�������ɫҺ�壬Ϊˮ��F��ǿ������ɫ��Ӧ����ʻ�ɫ��Ϊ�������ƣ�D��ij�־��д��ԵĽ����������Ҫ�ɷ֣�Ϊ������������K���ȷֽ�����ɺ���ɫ���壬Ϊ������������AΪ�ơ�
E���ܶ���С�����壬Ϊ������G�ǻ���ɫ�����壬Ϊ������B�dz�������ɫҺ�壬Ϊˮ��F��ǿ������ɫ��Ӧ����ʻ�ɫ��Ϊ�������ƣ�D��ij�־��д��ԵĽ����������Ҫ�ɷ֣�Ϊ������������K���ȷֽ�����ɺ���ɫ���壬Ϊ������������AΪ�ƣ�CΪ����IΪ�Ȼ�����JΪ�Ȼ�������
(1) DΪ��������������ѧʽΪFe3O4��
(2) FΪ�������ƣ�����ʽΪ![]() ��
��
(3) �Ȼ���������ϡ���ᷴӦ�����������Ӻ�һ�����������ӷ���ʽ��3Fe2+��4H+��NO3-===3Fe3+��NO��+2H2O��
(4) �������������������Ʒ�Ӧ�����Ȼ��ƺʹ������ƺ�ˮ������ʽΪ��Cl2��2NaOH===NaCl��NaClO��H2O��

����Ŀ���±���Ԫ�����ڱ���һ����, ��Ա��еĢ١�����Ԫ�أ���д���пհף�
���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0�� |
2 | �� | �� | �� | |||||
3 | �� | �� | �� | �� | �� | |||
4 | �� |
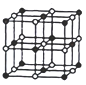
(1)�ߵ����ӽṹʾ��ͼ��__________��
(2)��������õĽ�����________(��дԪ�ط���)�����γɵ����ȶ���̬�⻯��Ļ�ѧʽ��_______��
(3)
(4)�ݡ����γɻ������к��еĻ�ѧ����________(�������Ӽ����������ۼ���)��
(5)1mol�ܵĵ����������ڵ��⻯����ȫ��Ӧ�����������ڱ�״���µ����Ϊ_____��
(6)д���ĵ�����ܵ�����������ˮ������Һ��Ӧ�Ļ�ѧ����ʽ_____________��