��Ŀ����
����Ŀ������������Ԫ��A��B��C��D��E��Ԫ�����ڱ��е�λ����ͼ��ʾ��A�ļ����Ӱ뾶��ͬ����Ԫ�ؼ������а뾶��С�ġ���ش��������⣺

(1)����D��Ԫ�����ڱ��е�λ�ã�_____________________________��
(2)��A��B��C��E����Ԫ������������Ӧ��ˮ������������ǿ����__________(д��ѧʽ����ͬ)���ǽ��������̬�⻯����ȶ�����_____________________��
(3)д�����������������ӵĽṹʽ_________________________
������ԭ���������8�����ӣ� ��C��E��ɵĻ�����
���𰸡��ڶ�����VIA HClO4 CH4 ![]()
��������
�ɶ���������Ԫ��A��B��C��D��E�����ڱ��е�λ��֪��B��C��D���ڵڶ����ڣ�A��E���ڵ������ڣ�AԪ�صļ����Ӱ뾶��ͬ����Ԫ�صļ���������С����A��AlԪ�أ�����֪BΪ̼Ԫ�ء�CΪNԪ�ء�DΪOԪ�ء�EΪCl���ݴ˽��
������������֪��
(1)DΪOԪ�أ�ԭ������Ϊ8����Ԫ�����ڱ��е�λ�ã��ڶ����ڢ�A�壬
�ʴ�Ϊ���ڶ����ڢ�A�壻
(2)��A. B. C.E����Ԫ������������Ӧ��ˮ������������ǿ����HClO4���ǽ��������̬�⻯����ȶ�����CH4��
�ʴ�Ϊ��HClO4��CH4��
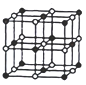
(3)���������������ӵĽṹʽ��������ԭ���������8�����ӣ���C��E��ɵĻ�����÷��ӽṹʽΪ��![]() ��
��
�ʴ�Ϊ��![]() ��
��