��Ŀ����
�����ʽṹ�����ʡ���15�֣�
����Ԫ�����ڱ��е�������Ԫ�ص����֪ʶ���ش��������⣺
��1����������Ԫ�صĻ�̬ԭ�ӵĵ����Ų���4s�����ֻ��1�����ӵ�Ԫ����_______�֣�
д��Cu+�ĺ�������Ų�ʽ_________��
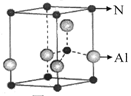
��2���������Ų����ɽ����ڱ����Ԫ�ػ��ֳ��������������Ԫ��������s����Ԫ����___________�֣�����d����Ԫ����________�֡�
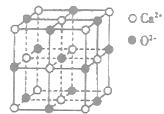
��3��CaO������ͼ��ʾ��CaO������Ca2������λ��Ϊ________��CaO����ɫ��ӦΪש��ɫ��������������ǵĻ����ﶼ���Է�����ɫ��Ӧ����ԭ����________��
��4���ɵ�������(KN3)�ȷֽ�ɵô� �������й�˵����ȷ����________����ѡ����ĸ����
�������й�˵����ȷ����________����ѡ����ĸ����
��5����������(TiO2)�dz��õġ����нϸߴ����Ժ��ȶ��ԵĹ������O2����������£��ɽ�CN��������CNO����CN���ĵ���ʽΪ________��CNO��������ԭ�ӵ��ӻ���ʽΪ____________��
��6����CrCl3��Һ�У�һ�������´������Ϊ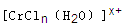 ��n��x��Ϊ���������������ӣ�����ͨ�������ӽ�����֬(R-H)���ɷ������ӽ�����Ӧ��
��n��x��Ϊ���������������ӣ�����ͨ�������ӽ�����֬(R-H)���ɷ������ӽ�����Ӧ��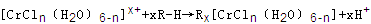
����0.0015mol ����Һ����R-H��ȫ�������к����ɵ���Ũ��Ϊ
����Һ����R-H��ȫ�������к����ɵ���Ũ��Ϊ
0.1200 mol/LNaOH��Һ25.00 mL����������ӵĻ�ѧʽΪ____________��
����Ԫ�����ڱ��е�������Ԫ�ص����֪ʶ���ش��������⣺
��1����������Ԫ�صĻ�̬ԭ�ӵĵ����Ų���4s�����ֻ��1�����ӵ�Ԫ����_______�֣�
д��Cu+�ĺ�������Ų�ʽ_________��
��2���������Ų����ɽ����ڱ����Ԫ�ػ��ֳ��������������Ԫ��������s����Ԫ����___________�֣�����d����Ԫ����________�֡�
��3��CaO������ͼ��ʾ��CaO������Ca2������λ��Ϊ________��CaO����ɫ��ӦΪש��ɫ��������������ǵĻ����ﶼ���Է�����ɫ��Ӧ����ԭ����________��

��4���ɵ�������(KN3)�ȷֽ�ɵô�
 �������й�˵����ȷ����________����ѡ����ĸ����
�������й�˵����ȷ����________����ѡ����ĸ����| A��NaN3��KN3�ṹ���ƣ�ǰ�߾����ܽ�С |
B������صľ����ṹ����ͼ��ʾ��ÿ�������з�̯2����ԭ�� |
| C�����ĵ�һ�����ܴ����� |
| D�����������º��ȶ�������Ϊ���ĵ縺��С |
��6����CrCl3��Һ�У�һ�������´������Ϊ
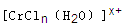 ��n��x��Ϊ���������������ӣ�����ͨ�������ӽ�����֬(R-H)���ɷ������ӽ�����Ӧ��
��n��x��Ϊ���������������ӣ�����ͨ�������ӽ�����֬(R-H)���ɷ������ӽ�����Ӧ��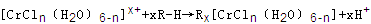
����0.0015mol
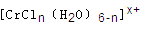 ����Һ����R-H��ȫ�������к����ɵ���Ũ��Ϊ
����Һ����R-H��ȫ�������к����ɵ���Ũ��Ϊ0.1200 mol/LNaOH��Һ25.00 mL����������ӵĻ�ѧʽΪ____________��
��1�� 3 ��1�֣� 1s22s22p63s23p63d10��1�֣�
��2�� 2 ��1�֣���8��1�֣�
��3�� 6����1�֣� ����̬�ĵ��Ӵ������ߵĹ��ԾǨ�������ϵ͵Ĺ��ʱ����һ�����������ʽ�ͷ������� ��2�֣����ֵ㣺����ԾǨ���ͷ�������
��4�� B C ��2��,��1�֣���ѡ���÷֣�
��5��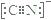 ��2�֣� sp�ӻ� ��2�֣�
��2�֣� sp�ӻ� ��2�֣�
��6�� [CrCl(H2O)5]2����2�֣�
��2�� 2 ��1�֣���8��1�֣�
��3�� 6����1�֣� ����̬�ĵ��Ӵ������ߵĹ��ԾǨ�������ϵ͵Ĺ��ʱ����һ�����������ʽ�ͷ������� ��2�֣����ֵ㣺����ԾǨ���ͷ�������
��4�� B C ��2��,��1�֣���ѡ���÷֣�
��5��
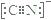 ��2�֣� sp�ӻ� ��2�֣�
��2�֣� sp�ӻ� ��2�֣���6�� [CrCl(H2O)5]2����2�֣�
�����������1��4s�����ֻ��1�����ӵ�Ԫ����K��Cr��Cu����Ԫ�أ�Cuԭ������Ϊ29����������Ų�ʽΪ��1s22s22p63s23p63d104s1��Cu+ʧȥ�������ӣ���ʧȥ��4s����ĵ��ӣ��ʴ�Ϊ��3��1s22s22p63s23p63d10��
��2��s�������ڢ�A����A�壬��������ֻ������Ԫ��λ�������壻d������Ԫ�����ڱ��ӵ����е���ʮ�У���8��Ԫ�أ��ʴ�Ϊ��2��8��
��3���۲�þ�������NaCl�ͣ�����λ��Ϊ6���������ܵ��������ٴӼ���̬ԾǨ�Ļ�̬ʱ�ͷ������������ض������Ĺ⣬�ʴ�Ϊ��6������̬�ĵ��Ӵ������ߵĹ��ԾǨ�������ϵ͵Ĺ��ʱ����һ�����������ʽ�ͷ�������
��4��A�����Ӱ뾶С��������Խ�������Ӱ뾶С�ڼ����ӣ���A����
B�����ݾ�̯�����㣬��ԭ��λ�ڶ�������ģ�ÿ����������ԭ��Ϊ8��1/8+1=2����B��ȷ��
C����ԭ�Ӽ۵����Ų�ʽΪ2s22p3��2p������������Ϊ�ȶ�����һ�����ܴ�����ԭ�ӣ���C��ȷ��
D�������������γɵ��������������仯ѧ�����ȶ�����������Ϊ�縺��С����D����
�ʴ�Ϊ��BC��
��5��Cԭ��������ĸ����ӣ��γ��ĶԹ��õ��Ӷԣ�Nԭ���������������ӣ��γ����Թ��õ��Ӷԣ�CN-�õ�һ�����ӣ���д������ʽΪ
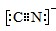 ����������COΪ�ȵ����壬��CO�ӻ�����һ�£�CO�ļ۵��Ӷ���=4/2=2��Ϊsp�ӻ���
����������COΪ�ȵ����壬��CO�ӻ�����һ�£�CO�ļ۵��Ӷ���=4/2=2��Ϊsp�ӻ�����6��c��H+��=c��NaOH��=0.1200mol?L-1��25.00mL��10-3=0.003mol�����ݷ���ʽ����0.0015mol[CrCln��H2O��6-n]x+��x=2����������������Cl-����Ϊ1����д������ʽ��[CrCl(H2O)5]2+.

��ϰ��ϵ�д�
�����Ŀ