��Ŀ����
��ÿ��1�֣���10�֣�������ѧ֪ʶ������ش��������⣺
��1������8�����ӣ�10�����ӵ�ԭ�ӵĻ�ѧ����__________��
��2�����������Ų�Ϊ4s24p1��ԭ�ӵĺ˵����Ϊ__________��
��3��ijԪ�ر���ѧ�ҳ�֮Ϊ������Ԫ���еġ�����֮��������ԭ�ӵ���Χ�����Ų���4s24p4����Ԫ�ص�������_________��
��4������VSEPRģ�ͣ�H3O+�ķ�������ṹΪ�� ��SO2������ṹΪ�� ��
��5�����ڱ�������õķǽ���Ԫ��ԭ�ӵĹ����ʾʽΪ__________ ��
( 6 ) ���Ȼ���������Ϊ���壬�۵�282��C���е�315�㣬��300��C������������������ˮ��Ҳ���������ѡ���ͪ���л��ܼ����ݴ��ж����Ȼ�������Ϊ________��
( 7 ) ijԪ�غ������������Ӳ㣬�����������Ǻ������������1/6��д����Ԫ��ԭ�ӵĵ����Ų�ʽ��__________��
( 8 )д����Ԫ�������ڱ��е�λ��__________����λ��__________����
��1������8�����ӣ�10�����ӵ�ԭ�ӵĻ�ѧ����__________��
��2�����������Ų�Ϊ4s24p1��ԭ�ӵĺ˵����Ϊ__________��
��3��ijԪ�ر���ѧ�ҳ�֮Ϊ������Ԫ���еġ�����֮��������ԭ�ӵ���Χ�����Ų���4s24p4����Ԫ�ص�������_________��
��4������VSEPRģ�ͣ�H3O+�ķ�������ṹΪ�� ��SO2������ṹΪ�� ��
��5�����ڱ�������õķǽ���Ԫ��ԭ�ӵĹ����ʾʽΪ__________ ��
( 6 ) ���Ȼ���������Ϊ���壬�۵�282��C���е�315�㣬��300��C������������������ˮ��Ҳ���������ѡ���ͪ���л��ܼ����ݴ��ж����Ȼ�������Ϊ________��
( 7 ) ijԪ�غ������������Ӳ㣬�����������Ǻ������������1/6��д����Ԫ��ԭ�ӵĵ����Ų�ʽ��__________��
( 8 )д����Ԫ�������ڱ��е�λ��__________����λ��__________����
��1��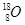 ��1�֣� ��2��31 ��1�֣� ��3���� ��1�֣� ��4�������Σ�1�֣���V�� ��1�֣�
��1�֣� ��2��31 ��1�֣� ��3���� ��1�֣� ��4�������Σ�1�֣���V�� ��1�֣�
��5��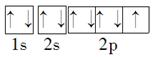 ��1�֣���6�� ���Ӿ��� ��1�֣� ��7�� 1s22s22p63s2��[Ne]3s2 ��1�֣�
��1�֣���6�� ���Ӿ��� ��1�֣� ��7�� 1s22s22p63s2��[Ne]3s2 ��1�֣�
��8�� �������ڢ�B�壨1�֣���d��1�֣�
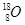 ��1�֣� ��2��31 ��1�֣� ��3���� ��1�֣� ��4�������Σ�1�֣���V�� ��1�֣�
��1�֣� ��2��31 ��1�֣� ��3���� ��1�֣� ��4�������Σ�1�֣���V�� ��1�֣� ��5��
 ��1�֣���6�� ���Ӿ��� ��1�֣� ��7�� 1s22s22p63s2��[Ne]3s2 ��1�֣�
��1�֣���6�� ���Ӿ��� ��1�֣� ��7�� 1s22s22p63s2��[Ne]3s2 ��1�֣���8�� �������ڢ�B�壨1�֣���d��1�֣�
�����������1���ڱ�ʾԭ�����ʱԪ�ط��ŵ����½DZ�ʾ�����������ϽDZ�ʾ������������8�����ӣ�10�����ӵ�ԭ�ӵĻ�ѧ����
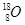 ��
����2�����������Ų�Ϊ4s24p1��ԭ�ӵĺ˵����Ϊ2+8+18+3��31��
��3��ijԪ�ر���ѧ�ҳ�֮Ϊ������Ԫ���еġ�����֮��������ԭ�ӵ���Χ�����Ų���4s24p4������ԭ��������2+8+18+6��34�����Ը�Ԫ�ص�����������
��4��H3O+�м۲���Ӷ�����4��������ԭ�Ӻ���1�Թ¶Ե��ӣ����Է�������ṹΪ�����Σ�SO2���Ӽ۲���Ӷ�����3��������ԭ�Ӻ���1�Թ¶Ե��ӣ���������ṹΪV�Ρ�
��5�����ڱ�������õķǽ���Ԫ����FԪ�أ�����ԭ�ӵĹ����ʾʽΪ
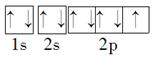 ��
����6�����Ȼ���������Ϊ���壬�۵�282��C���е�315�㣬��300��C������������������ˮ��Ҳ���������ѡ���ͪ���л��ܼ����ݴ��ж����Ȼ�������Ϊ���Ӿ��塣
��7��ijԪ�غ������������Ӳ㣬�����������Ǻ������������1/6������������������x�����ԣ�10+x����6��x�����x��2����˸�Ԫ��ԭ�ӵĵ����Ų�ʽ��1s22s22p63s2��[Ne]3s2��
��8����Ԫ�������ڱ��е�λ�õ������ڢ�B�塣�������������ڰ��չ���ԭ�����ͨ����ӵĹ�����ƣ����Ը�Ԫ��λ��d����

��ϰ��ϵ�д�
�����Ŀ
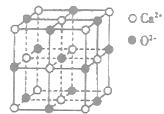
 �������й�˵����ȷ����________����ѡ����ĸ����
�������й�˵����ȷ����________����ѡ����ĸ����
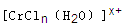 ��n��x��Ϊ���������������ӣ�����ͨ�������ӽ�����֬(R-H)���ɷ������ӽ�����Ӧ��
��n��x��Ϊ���������������ӣ�����ͨ�������ӽ�����֬(R-H)���ɷ������ӽ�����Ӧ��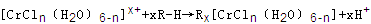
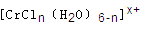 ����Һ����R-H��ȫ�������к����ɵ���Ũ��Ϊ
����Һ����R-H��ȫ�������к����ɵ���Ũ��Ϊ ������ĿΪ____ __________________.
������ĿΪ____ __________________. g/cm
g/cm �������ӵ�����Ϊ
�������ӵ�����Ϊ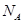 �����߳�a=________cm�����ú�
�����߳�a=________cm�����ú�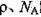 �ļ���ʽ��ʾ��
�ļ���ʽ��ʾ��


 ��������ܲ����ȫ������
��������ܲ����ȫ������