��Ŀ����
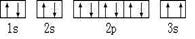
[��ѧ�����ʽṹ������]��13�֣�
���ֽ��˶����ݴ������������ϻ���Ļ��ʹ���˷�������ܣ�LED����Ŀǰ����LED��Ƭ�����ʻ�����GaAs���黯�أ���InGaN���������أ�Ϊ�����黯����Ϊ�������뵼�壬����Ϊ��������ĵ����������������١�
��֪�黯�صľ����ṹ��ͼ��ʾ����ش��������⣺

��1������˵����ȷ���� ������ĸ��ţ���
a���黯�ؾ����ṹ��NaCl��ͬ b����һ�����ܣ�As��Ga
c���縺�ԣ�As��Ga d���黯�ؾ����к�����λ��
e��GaP��GaAs��Ϊ�ȵ�����
��2��AsH3�ռ乹��Ϊ__ __���黯�ؿ���(CH3)3Ga ��AsH3��700��ʱ�Ƶã�(CH3)3Ga����ԭ�ӵ��ӻ���ʽΪ ��
�� ����ͭ�ĵ����Խ����������ӽ����еĵڶ�λ���������ڵ�����ҵ��
��3��Cu�ļ۵����Ų�ʽΪ__________������ͽ���ͭ�ܵ����ԭ�� ��
��4��������ͭ��Һ��ͨ������İ�����С�����������յõ�����ɫ��[Cu(NH3)4]SO4���壬������
���еĻ�ѧ������ͨ���ۼ��⣬���� �� ����֪NF3��NH3�Ŀռ乹�Ͷ��������Σ���NF3������Cu2+�γ������ӣ���ԭ���� ��
���ֽ��˶����ݴ������������ϻ���Ļ��ʹ���˷�������ܣ�LED����Ŀǰ����LED��Ƭ�����ʻ�����GaAs���黯�أ���InGaN���������أ�Ϊ�����黯����Ϊ�������뵼�壬����Ϊ��������ĵ����������������١�
��֪�黯�صľ����ṹ��ͼ��ʾ����ش��������⣺

��1������˵����ȷ���� ������ĸ��ţ���
a���黯�ؾ����ṹ��NaCl��ͬ b����һ�����ܣ�As��Ga
c���縺�ԣ�As��Ga d���黯�ؾ����к�����λ��
e��GaP��GaAs��Ϊ�ȵ�����
��2��AsH3�ռ乹��Ϊ__ __���黯�ؿ���(CH3)3Ga ��AsH3��700��ʱ�Ƶã�(CH3)3Ga����ԭ�ӵ��ӻ���ʽΪ ��
�� ����ͭ�ĵ����Խ����������ӽ����еĵڶ�λ���������ڵ�����ҵ��
��3��Cu�ļ۵����Ų�ʽΪ__________������ͽ���ͭ�ܵ����ԭ�� ��
��4��������ͭ��Һ��ͨ������İ�����С�����������յõ�����ɫ��[Cu(NH3)4]SO4���壬������
���еĻ�ѧ������ͨ���ۼ��⣬���� �� ����֪NF3��NH3�Ŀռ乹�Ͷ��������Σ���NF3������Cu2+�γ������ӣ���ԭ���� ��
��1��b c d e��2�֣� ��2�������ͣ�2�֣� sp2 ��2�֣�
��3��3d104s1��2�֣� ����ͭ�������ɵ��ӣ�ͨ��������ӵĶ����ƶ�����������1�֣�
��4����λ�������Ӽ���2�֣� F��H�縺�Դ�������������ǿ�����Ӷ�ǿ��ƫ����F����Nԭ�ӵŵ��ӶԲ�����Cu2+�γ���λ�� ��2�֣�
��3��3d104s1��2�֣� ����ͭ�������ɵ��ӣ�ͨ��������ӵĶ����ƶ�����������1�֣�
��4����λ�������Ӽ���2�֣� F��H�縺�Դ�������������ǿ�����Ӷ�ǿ��ƫ����F����Nԭ�ӵŵ��ӶԲ�����Cu2+�γ���λ�� ��2�֣�
�������������1��a��GaAs������As�ֲ��ھ������ģ�Ga�ֲ��ڶ�������ģ���NaCl���������ӷֱ�λ�ھ����Ķ��㡢�����Լ�������ģ����߽ṹ��ͬ����a����b��ͬ����Ԫ�ش����ҵ�һ������������ƣ�����VA����ڵ�IIIA�壬��IIA����ڵ�IIIA�壬�������һ�����ܣ�As��Ga����b��ȷ��c��ͬ����Ԫ�ش����ҵ縺����������縺�ԣ�As��Ga����c��ȷ��d����۵�������5��������3����Ҫ����8�����ȶ��ṹ���� �黯�ؾ����к�����λ������d��ȷ��e��GaP�ļ۲����Ϊ3+5=8��SiC�ļ۲����Ϊ4+4=8��GaAs�۲������Ϊ3+5=8����Ϊ�ȵ����壬��e��ȷ���ʴ�Ϊ��bcde����2��AsH3�к���3���ļ���1���µ��Ӷԣ�Ϊ�����Σ���CH3��3Ga��Ga�γ�3���ļ���û�йµ��Ӷԣ�Ϊsp2�ӻ���
��3��Cu��ԭ��������29�����ݺ�������Ų����ɿ�֪ͭ�ļ۵����Ų�ʽΪ3d104s1�����ڽ���ͭ�������ɵ��ӣ�ͨ��������ӵĶ����ƶ�������������˽���ͭ�ܵ��磻
��4���������ʵĽṹ��֪��ͭ���������������֮�仹�������Ӽ���ͭ�����백�����Ӽ仹������λ��������F��H�縺�Դ�������������ǿ�����Ӷ�ǿ��ƫ����F����Nԭ�ӵŵ��ӶԲ�����Cu2+�γ���λ����

��ϰ��ϵ�д�
�����Ŀ
 ��Υ���� ԭ����
��Υ���� ԭ����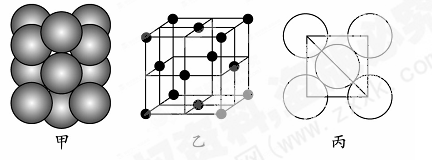
 �������й�˵����ȷ����________����ѡ����ĸ����
�������й�˵����ȷ����________����ѡ����ĸ����
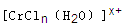 ��n��x��Ϊ���������������ӣ�����ͨ�������ӽ�����֬(R-H)���ɷ������ӽ�����Ӧ��
��n��x��Ϊ���������������ӣ�����ͨ�������ӽ�����֬(R-H)���ɷ������ӽ�����Ӧ��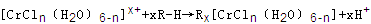
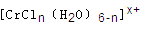 ����Һ����R-H��ȫ�������к����ɵ���Ũ��Ϊ
����Һ����R-H��ȫ�������к����ɵ���Ũ��Ϊ
 4FeCl2+N2O��+6HCl+H2O
4FeCl2+N2O��+6HCl+H2O ����������
����������
 �ͺ�ɫ��HD���ֻ����
�ͺ�ɫ��HD���ֻ���� ��Bԭ�Ӳ�ȡ���ӻ��������Ϊ ��
��Bԭ�Ӳ�ȡ���ӻ��������Ϊ �� ���ӵĿռ乹��Ϊ ��
���ӵĿռ乹��Ϊ �� ��������
��������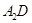 ,ԭ���� ��
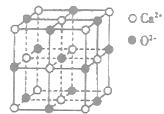
,ԭ���� ��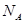 ��ʾ����٤�������������ı߳�Ϊa�������ĸ�Ϊh�������ܶȿɱ�ʾΪ g/cm3������ֻ��r��
��ʾ����٤�������������ı߳�Ϊa�������ĸ�Ϊh�������ܶȿɱ�ʾΪ g/cm3������ֻ��r��
