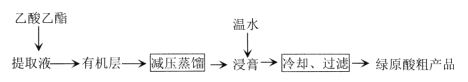
��Ŀ����
����Ŀ�������仯�����ڹ�ũҵ������������������Ҫ���á����������գ�
�ϳɰ���ҵ�У�N2(g) + 3H2(g) ![]() 2NH3(g) + Q(Q��0)���仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ�����
2NH3(g) + Q(Q��0)���仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ�����
t/�� | 200 | 300 | 400 |
K | K1 | K2 | 0.5 |
(1)�ԱȽ�K1��K2�Ĵ�С��K1 ________K2����д����������=������������
(2) 400��ʱ����Ӧ2NH3(g) ![]() N2(g) + 3H2(g)�Ļ�ѧƽ�ⳣ����ֵΪ_____________��
N2(g) + 3H2(g)�Ļ�ѧƽ�ⳣ����ֵΪ_____________��
�����NH3��N2��H2�����ʵ���Ũ�ȷֱ�Ϊ3 mol/L��2 mol/L��1 mol/Lʱ����÷�Ӧ��(N2)(��) ___________��(N2)(��)����д����������=������������
(3)���ܱպ��ݵ������У���������Ϊ�ϳɰ���Ӧ�ﵽƽ������ݵ���____________��
a����(N2)������=3�� (H2)���棩 b�����������ܶȱ��ֲ���
c��������ѹǿ���ֲ��� d��N2��H2��NH3��Ũ��֮��Ϊ1��3��2
(4)��������Ϊ�����������������������ö��ַ�������д������ϳɰ���ҵ��Ϊʵ������������ȡ�Ĵ�ʩ��__________________________��__________________________��
(5)0.1 mol/L��(NH4)2SO4ˮ��Һ�и�����Ũ���ɴ�С��˳����_____________________���ڸ���Һ�м��������������壬��Һ��NH4+��Ũ��_______������������������С������������������ԭ����_________________________________________________________��
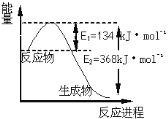
(6)��ͼ��1 mol NO2��1 mol CO��Ӧ����CO2��NO�����������仯ʾ��ͼ����д��NO2�� CO��Ӧ���Ȼ�ѧ����ʽ _____________________________________________��
���𰸡��� 2 �� c ����20MPa~50MPa��ѹ ��ʱ������Һ����ȥ c(NH4+)> c(SO42-)> c(H+)> c(OH-) ���� NH4+ ˮ������ԣ�Al3+ˮ��Ҳ�����ԣ���������ƣ�c(NH4+)���� NO2(g)+CO(g)=CO2(g)+NO(g) + 234kJ/mol
��������
(1)�ϳɰ�N2(g) + 3H2(g) ![]() 2NH3(g) + Q(Q��0)������Ϊ���ȷ�Ӧ�����¶�����ƽ�������ƶ���ƽ�ⳣ��Kֵ��С����K1 ��K2��
2NH3(g) + Q(Q��0)������Ϊ���ȷ�Ӧ�����¶�����ƽ�������ƶ���ƽ�ⳣ��Kֵ��С����K1 ��K2��
(2) 400��ʱ��N2(g) + 3H2(g) ![]() 2NH3(g)��ƽ�ⳣ��K=0.5����2NH3(g)
2NH3(g)��ƽ�ⳣ��K=0.5����2NH3(g) ![]() N2(g) + 3H2(g) ��ƽ�ⳣ��Ϊ
N2(g) + 3H2(g) ��ƽ�ⳣ��Ϊ![]() =
=![]() =2�������NH3��N2��H2�����ʵ���Ũ�ȷֱ�Ϊ3 mol/L��2 mol/L��1 mol/Lʱ��Qc=
=2�������NH3��N2��H2�����ʵ���Ũ�ȷֱ�Ϊ3 mol/L��2 mol/L��1 mol/Lʱ��Qc=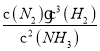 =
=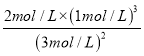 =
=![]() ��K����Ӧ������У�����(N2)(��) ����(N2)(��) ��
��K����Ӧ������У�����(N2)(��) ����(N2)(��) ��
(3)���ܱպ��ݵ������У�a����(N2)������=3�� (H2)���棩����ͬ���ʱ�ʾ���������ʲ����ڻ�ѧ������֮�ȣ���Ӧû�дﵽƽ��״̬��a��ѡ��b�����������ܶ�=![]() ���������������غ�m�Ƕ������������������������ܶ�ʼ���Ǹ���ֵ���ʻ��������ܶȱ��ֲ��䣬����˵����Ӧ�ﵽƽ��״̬��b��ѡ��c��ͬ��ͬѹ�£������ڵ�ѹǿ����������ʵ��������ȣ��÷�Ӧ���������������Ŀ��С��������������ʵ����Ǹ��仯����ѹǿ�Ǹ���������ѹǿ���ֲ��䣬˵����Ӧ�ﵽƽ��״̬��cѡ��d�������ʵ����ʵ���Ũ�ȱ��ֲ���������ж����ݣ�N2��H2��NH3��Ũ��֮��Ϊ1��3��2�������ʵ�Ũ�ȱȵ��ڻ�ѧ������֮�Ȳ���˵���ﵽƽ�⣬d��ѡ��������Ϊ�ϳɰ���Ӧ�ﵽƽ������ݵ�ѡc��
���������������غ�m�Ƕ������������������������ܶ�ʼ���Ǹ���ֵ���ʻ��������ܶȱ��ֲ��䣬����˵����Ӧ�ﵽƽ��״̬��b��ѡ��c��ͬ��ͬѹ�£������ڵ�ѹǿ����������ʵ��������ȣ��÷�Ӧ���������������Ŀ��С��������������ʵ����Ǹ��仯����ѹǿ�Ǹ���������ѹǿ���ֲ��䣬˵����Ӧ�ﵽƽ��״̬��cѡ��d�������ʵ����ʵ���Ũ�ȱ��ֲ���������ж����ݣ�N2��H2��NH3��Ũ��֮��Ϊ1��3��2�������ʵ�Ũ�ȱȵ��ڻ�ѧ������֮�Ȳ���˵���ﵽƽ�⣬d��ѡ��������Ϊ�ϳɰ���Ӧ�ﵽƽ������ݵ�ѡc��
(4)�ϳɰ���ҵ��Ϊʵ������������߰��IJ��ʣ����Դ�ƽ���ƶ��Ƕȿ��ǣ��ʵ�����ѹǿ��ƽ�������ƶ����ʵ����ͷ�Ӧ���¶ȣ�ƽ�������ƶ�����ʱ������Һ�����������ϵ��ƽ�������ƶ�����������߷�Ӧ���ת���ʶ���߰����ʣ� ������ȡ�Ĵ�ʩ������20MPa~50MPa��ѹ����ʱ������Һ�����룻
(5)0.1 mol/L��(NH4)2SO4ˮ��Һ��NH4+����ˮ����Һ�����ԣ�����ˮ�������ģ�c(NH4+)ԼΪc(SO42-)���������ʸ�����Ũ���ɴ�С��˳����c(NH4+)> c(SO42-)> c(H+)> c(OH-)���ڸ���Һ�м��������������壬Al3+ˮ��Ҳ�����ԣ���������ƣ�c(NH4+)����
(6)���ݷ�Ӧ��������ͼ���÷�ӦΪ���ȷ�Ӧ���ҷų���ֵΪ368kJ/mol-134kJ/mol=234kJ/mol��NO2�� CO��Ӧ���Ȼ�ѧ����ʽ��NO2(g)+CO(g)=CO2(g)+NO(g) + 234kJ/mol��