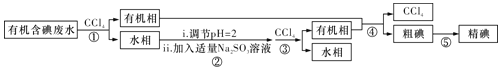
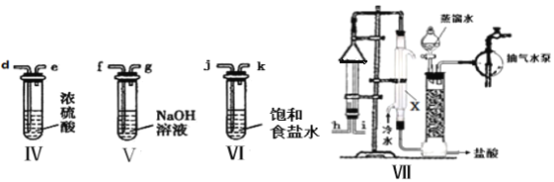
��Ŀ����
����Ŀ���ӹ����������仯��������������������ж����˾�����á�
��1���Ŵ��й��Ĵ���֮һ��ָ����������Ȼ��ʯ�Ƴɵģ�����Ҫ�ɷ���_________________��
A��Fe B��FeO C��Fe3O4 D��Fe2O3
��2�����ִ����������У�������FeCl3��ʴͭ��ԭ������ӡˢ��·�壬д����ԭ���Ļ�ѧ����ʽ_________________________________��
��3��ʵ�������̷�FeSO4��xH2O����FeSO4��ҺʱΪ�˷�ֹFeSO4��Һ���ʣ����������м������ۣ���ԭ����_____________________ (�����ӷ���ʽ��ʾ)��
��4��ijͬѧ��ȡ2 mL FeSO4��Һ������1��KSCN��Һ���ټ��뼸����ˮ����Һ��죬˵��Cl2�ɽ�Fe2���������̷���Һ����ˮ��Ӧ�����ӷ���ʽΪ_______��
��5��ͬѧ����Ϊ��ͬѧ��ʵ�鲻���Ͻ�����ͬѧ��2 mL FeSO4 ��Һ���ȼ���0.5 mLú�ͣ�����Һ�������μ���1��KSCN��Һ�ͼ�����ˮ����Һ��죬ú�͵�������_____________��
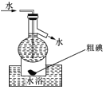
��6��Ϊ�ⶨij�̷�FeSO4��xH2O�нᾧˮ��������ʯӢ�����ܣ������˿���K1��K2������Ϊװ��A�����أ���Ϊm1 g������Ʒװ��ʯӢ�������У��ٴν�װ��A���أ���Ϊ m2 g������ͼ���Ӻ�װ�ý���ʵ�飺
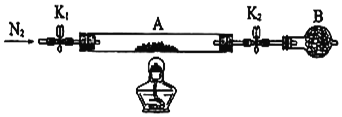
������B��������____________��
�ڽ�����ʵ�����������ȷ����__________�����ţ����ظ������������裬ֱ��A���أ���Ϊm3 g��
a����ȼ�ƾ��ƣ�����
b��Ϩ��ƾ���
c���ر�K1��K2
d����K1��K2������ͨ��N2
e������A
f����ȴ������
�۸���ʵ���¼�������̷�FeSO4��xH2O��ѧʽ�нᾧˮ��Ŀx=_______________________����ʽ��ʾ����
���𰸡�C 2Fe Cl3+Cu===2FeCl2+CuCl2 2Fe3��+Fe==3 Fe2�� 2Fe2��+ Cl2==2Fe2��+2Cl- ��������(�ų�������ʵ���Ӱ��) ���θ���� dabcfe 76(m2-m3)/9(m3-m1)
��������
��1���������������д��ԣ�
��2���Ȼ�����ͭ��Ӧ�����Ȼ��������Ȼ�ͭ��
��3�����������������������ӣ������ۿ��Է�ֹ�������ӱ�������
��4���������Խ�������������Ϊ�����ӣ�
��5��ú����������ֹ�������ӱ�����������
��6���̷�����ʧȥ�ᾧˮ�������������������ͽᾧˮ���������ɼ����x��ֵ��
��1���������������д��ԣ�
��ѡC��
��2���Ȼ�����ͭ��Ӧ�����Ȼ��������Ȼ�ͭ������ʽΪ��![]() ��
��
����![]() ��
��
��3�����������������������ӣ������ۿ��Է�ֹ�������ӱ����������ӷ���ʽΪ��![]() ��
��
����![]() ��
��
��4��������������������Ϊ�����ӣ����ӷ���ʽΪ��![]() ,
,
����![]() ��
��
��5��ú���ܶȱ�ˮС����ˮ�ֲ��ú�����ϲ㣬����������ֹ�������ӱ�����������
�ʴ�Ϊ����������(�ų�������ʵ���Ӱ��)��
��6��������B�����������θ���ܣ��ʴ�Ϊ�����θ���ܣ�
��Ϊ��ֹ�����е�O2���̷���������K1��K2ͨ�뵪��������ʹ�̷�ʧȥ�ᾧˮ������һ��ʱ���ֹͣ���ر�K1��K2��Ȼ����ȴ�����¡�����A���������ظ�����ֱ�����أ�����ʵ�����������ȷ����Ϊ��dabcfe���ʴ�Ϊ��dabcfe��
��![]() ����Ϊ
����Ϊ![]() ��FeSO4���ʵ���Ϊ
��FeSO4���ʵ���Ϊ![]() ���ᾧˮ������Ϊ
���ᾧˮ������Ϊ![]() �����ʵ���Ϊ
�����ʵ���Ϊ![]() ����
����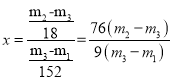 ��
��
�ʴ�Ϊ��![]() ��
��