��Ŀ����
����Ŀ������(N2H4)�ʹ�������(NaH2PO2)������ǿ��ԭ��.�����Ź㷺����;��
(1)��֪:��N2H4(l)+O2(g)=N2(g)+2H2O(g) ��H=-621.5 kJ��mol-1
��N2O4(l)-=N2(g)+2O2(g) ��H2=+204.3 kJ��mol-1
����ȼ�ϵ�ȼ�շ�ӦΪ2N2H4(l)+N2O4(l)=3N2(g)+4H2O(g) ��H=_____.
(2)��֪��ӦN2H4(g)+ 2Cl2(g)![]() N2(g)+4HCl(g),T��Cʱ,��V L�����ܱ������м���2 mol N2H4(g)��4 mol Cl2(g) ,���Cl2��HCl��Ũ����ʱ��Ĺ�ϵ��ͼ��ʾ��
N2(g)+4HCl(g),T��Cʱ,��V L�����ܱ������м���2 mol N2H4(g)��4 mol Cl2(g) ,���Cl2��HCl��Ũ����ʱ��Ĺ�ϵ��ͼ��ʾ��
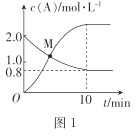
��0~ 10 min��,��N2(g)��ʾ��ƽ����Ӧ����v(N2)=_______��
��M��ʱ,N2H4��ת����Ϊ______(��ȷ��0.1)%��
��T ��Cʱ,�ﵽƽ�������������м���1.2 mol N2H4(g)��0.4 mol Cl2(g)��0. 8 mol N2 (g)��1.2 mol HCl(g) ,��ʱƽ��______(���������ƶ����������ƶ����������ƶ���)��
(3)���ڶ���������,������(P4)��ʯ�����̼������Һһͬ��������黯��Ӧ�����Ƶ�NaH2PO2,ͬʱ����������(PH3)����,�÷�Ӧ�Ļ�ѧ����ʽΪ________________��
�ڴ�����(H3PO2)��һԪ�ᣬ������.1.0 mol��L-1��NaH2PO2��ҺpHΪ8,��������Ka=___________��
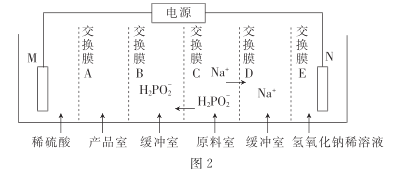
���ô�������ͨ�����������Ʊ�������.װ����ͼ2��ʾ������ĤA����____(������������������������)����Ĥ,�缫N�ĵ缫��ӦʽΪ______,����·������3.8528��105���ص���ʱ.�Ƶô���������ʵ���Ϊ_____ (һ�����ӵĵ���Ϊ 1.6��10- 19����,NA��ֵԼΪ6. 02�� 1023)��
���𰸡�-1038.7kJmol-1 0.06mol/(Lmin) 33.3 �����ƶ� 2P4+3Ca(OH)2+3Na2CO3+6H2O==== 6NaH2PO2+2PH3��+3CaCO3 1.0��10-2 ������ 2H2O+2e-=H2��+2OH- 4mol
��������
(1)���ݸ�˹���ɣ���Ӧ����2+�ڼ��ɵû��ȼ�ϵ�ȼ�շ�Ӧ2N2H4(1)+N2O4(l)===3N2(g)+4H2O(g)��H=(-621.5��2+204.3)kJmol-1=-1038.7kJmol-1��
(2)����ͼʾ��Cl2��Ϊ��Ӧ��Ũ�����С��HCl��Ϊ������Ũ��������
�١�c(Cl2)=(2-0.8)mol/L=1.2mol/L��v(Cl2)=![]() =0.12mol/(Lmin)�����ݷ�Ӧ���������ϵ�������ȿɵ�v(N2)=
=0.12mol/(Lmin)�����ݷ�Ӧ���������ϵ�������ȿɵ�v(N2)=![]() v(Cl2)=0.06mol/(Lmin)��
v(Cl2)=0.06mol/(Lmin)��
�ھ�ͼ��֪��Ӧ��ʼʱc(Cl2)=2mol/L��Ͷ��Ϊ2 mol N2H4(g)��4 mol Cl2(g) ����ʼʱc(N2H4)=1mol/L���������Ϊ2L����M�� N2H4��ת����Ϊx mol/L��������ʽ��
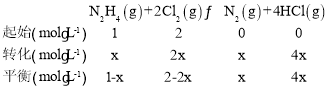
M��Cl2��HCl��Ũ����ȣ���2-2x=4x���x=![]() mol/L����N2H4��ת����Ϊ
mol/L����N2H4��ת����Ϊ =33.3%��
=33.3%��
�۾�ͼ��֪��Ӧƽ��ʱc(Cl2)=0.8mol/L����ʼc(Cl2)=2mol/L��c(N2H4)=1mol/L������ݷ�Ӧ����ʽN2H4(g)+ 2Cl2(g)![]() N2(g)+4HCl(g)��֪����Ӧ������c(Cl2)=1.2mol/L������c(N2H4)=0.6mol/L������c(N2)=0.6mol/L��c(HCl)=2.4mol/L������ƽ��ʱ�����ʵ�Ũ��Ϊc(Cl2)=0.8mol/L��c(N2H4)=0.4mol/L��c(N2)=0.6mol/L��c(HCl)=2.4mol/L������¶��µ�ƽ�ⳣ��K=
N2(g)+4HCl(g)��֪����Ӧ������c(Cl2)=1.2mol/L������c(N2H4)=0.6mol/L������c(N2)=0.6mol/L��c(HCl)=2.4mol/L������ƽ��ʱ�����ʵ�Ũ��Ϊc(Cl2)=0.8mol/L��c(N2H4)=0.4mol/L��c(N2)=0.6mol/L��c(HCl)=2.4mol/L������¶��µ�ƽ�ⳣ��K=![]() =77.76��
=77.76��
ƽ�������������м���1.2molN2H4(g)��0.4 mol Cl2(g)��0.8molN2(g)��1.2molHCl(g)�������ʵ�Ũ�ȱ�Ϊc(Cl2)=1mol/L��c(N2H4)=1mol/L��c(N2)=1mol/L��c(HCl)=3mol/L����ʱQc=![]() =81>K������ƽ�������ƶ���
=81>K������ƽ�������ƶ���
(3)�ٸ÷�Ӧ�з�Ӧ����P4��Ca(OH)2��Na2CO3�ȡ���������NaH2PO2��PH3�ȣ��ݴ˿�֪�÷�Ӧ��PԪ�ػ��ϼۼ������ֽ��ͣ������绯������1�ۣ�����3�ۣ���NaH2PO2��PH3��ϵ����Ϊ3��1���ٽ��Ԫ���غ��֪����ʽΪ��2P4+3Ca(OH)2+3Na2CO3+6H2O==== 6NaH2PO2+2PH3��+3CaCO3��
�ڴ�����(H3PO2)��һԪ�ᣬ1.0molL-1�� NaH2PO2��Һˮ�ⷽ��ʽΪ��H2PO2-+H2O=H3PO2![]() =1.0��10-2��
=1.0��10-2��
�۸���ͼʾ��֪����Ʒ�Ҳ��������ᣬ��缫MӦΪ�������ˮ�е�OH-��ʣ��H+ͨ������ĤA�����Ʒ�ң����Խ���ĤAΪ�����ӽ���Ĥ���缫NΪ�������H+���缫N�ĵ缫��ӦʽΪ2H2O+2e-=H2��+2OH-����·������3.8528��105���ص���ʱ��ת�Ƶĵ��ӵ����ʵ���Ϊ![]() =4mol�����������һ������ɣ������Ӵ�һ������ɣ����Ե�������ת��1mol��������1mol�����ᣬ��ת��4mol����ʱ����4mol�����ᡣ
=4mol�����������һ������ɣ������Ӵ�һ������ɣ����Ե�������ת��1mol��������1mol�����ᣬ��ת��4mol����ʱ����4mol�����ᡣ

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ���û���̿��ԭ��������������йط�ӦΪ��C(s)+ 2NO��g��![]() N2��g��+ CO2��g����ij�о�С����ij�ܱյ������������������������䣬��������������Բ��ƣ��м���NO�������Ļ���̿�����£�T1�棩�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
N2��g��+ CO2��g����ij�о�С����ij�ܱյ������������������������䣬��������������Բ��ƣ��м���NO�������Ļ���̿�����£�T1�棩�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
ʱ��min/ Ũ��mol��L-1 | NO | N2 | CO2 |
0 | 1.00 | 0 | 0 |
10 | 0.58 | 0.21 | 0.21 |
20 | 0.40 | 0.30 | 0.30 |
30 | 0.40 | 0.30 | 0.30 |
40 | 0.32 | 0.34 | 0.17 |
50 | 0.32 | 0.34 | 0.17 |
��1�� 10 min��20 min��ʱ����ڣ���CO2��ʾ�ķ�Ӧ����Ϊ____��
��2��������÷�Ӧ��ƽ�ⳣ����ֵK=________��
��3�� ���и�������Ϊ�жϸ÷�Ӧ�ﵽƽ��״̬����____ ���������ĸ����
A��������ѹǿ���ֲ��� B��2v����NO��= v����N2��
C��������CO2������������� D�����������ܶȱ��ֲ���
��4��30 minʱ�ı�ijһ��������Ӧ���´ﵽƽ�⣬��ı������������_____________��
��5��һ���¶��£�����NO����ʼŨ��������NO��ƽ��ת����__________ ����������
����Ŀ�����ᣨH2C2O4����һ�ֶ�Ԫ���ᣬ�㷺�ֲ��ڶ�ֲ�����С�
��1�������ڲ����ۻ������ǵ��½�ʯ����Ҫ�ɷ��Dz���ƣ��γɵ�ԭ��֮һ�����о����֣�EDTA��һ���ܽ�Ͻ������ӵ��Լ�����һ�������¿�����Ч�ܽ��ʯ���û�ѧƽ��ԭ��������ԭ��_______________��
��2����֪��0.1 mol��L��1KHC2O4��Һ�����ԡ�����˵����ȷ����_______������ĸ��ţ���
a. 0.1 mol��L��1KHC2O4��Һ�У�c(K+) + c(H+) = c(HC2O4-) + 2c(C2O42-) + c(OH-)
b. 0.1 mol��L��1KHC2O4��Һ�У�c(K+) > c(HC2O4-) > c(C2O42-) > c(H2C2O4)
c. Ũ�Ⱦ�Ϊ0.1 mol��L��1KHC2O4��K2C2O4�Ļ����Һ�У�2c(K+) = c(HC2O4-) + c(C2O42-)
d. 0.1 mol/L KHC2O4��Һ�еμӵ�Ũ��NaOH��Һ�����ԣ�c(K+) > c(Na+)
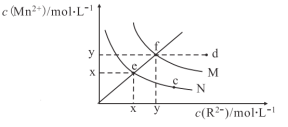
��3�����ò����Ʊ�������������(FeC2O4��xH2O)�����̼���ֲⶨ�������£�
![]()
��֪��i. pH>4ʱ��Fe2+�ױ���������
ii. �������ʵ��ܽ��(g /100g H2O)����
FeSO4��7H2O | (NH4)2SO4 | FeSO4��(NH4)2SO4��6H2O | |
20�� | 48 | 75 | 37 |
60�� | 101 | 88 | 38 |
����ϡ�������ҺpH��1��2��Ŀ���ǣ�_____________��______________��
�����ȹ��˵�ԭ���ǣ�_______________��
��������ԭ�ζ��������ڲⶨ�������������Ħ������(M)����ȡa g����������������ϡ�����У���b mol��L��1�ĸ�����ر�Һ�ζ�������ζ��յ�ʱ�����ĸ������VmL����M =__________��(��֪�����ַ�Ӧ����ΪMn2+��Fe3+��CO2)