��Ŀ����
��18�֣�A��B��C��D��E���ֶ�����Ԫ�أ�ԭ��������������AԪ�صĵ�������Ȼ����������壬E�����뱣����ú���С�A��B��C�ֱɵ�������ȵĻ�����M��N����M��һ���ܲ�������ЧӦ������л��������ʣ�DԪ�������������Ǵ�����������3�����ش��������⣺
��1��N�ĵ���ʽΪ ��M�����к��� ������ԡ��Ǽ��ԡ�����
��2��D��E��1:1�γɵĻ�������ˮ��Ӧ�Ļ�ѧ��Ӧ����ʽ
(3)A��B��C��D���γ������Ӻ������Ӹ�������1��1�����ӻ�����X��A��D��E���γɻ�����Y��X��Y�����ʵ���֮��1��2���ȷ�Ӧ��д����Ӧ�Ļ�ѧ����ʽ
��Ӧ��ˮ��Һ�� ����ᡱ������С�����,ԭ���� �������ӷ���ʽ��ʾ��
��4����N��D�ĵ��ʡ�KOH��Һ����ԭ��أ����������C�ĵ��ʡ����为����ӦΪ ��һ��ʱ�����ҺpH ���������С�����䡱����
 ��5����һ���¶��£���4 mol C���ʺ�12 mol A����ͨ�뵽���Ϊ2L���ܱ������У�������Ӧ��2 min�ﵽƽ��״̬ʱ��A���ʵ�ת������50%������A���ʱ�ʾ�÷�Ӧ��ƽ������Ϊ �����¶��µ�ƽ�ⳣ��ΪK= ��
��5����һ���¶��£���4 mol C���ʺ�12 mol A����ͨ�뵽���Ϊ2L���ܱ������У�������Ӧ��2 min�ﵽƽ��״̬ʱ��A���ʵ�ת������50%������A���ʱ�ʾ�÷�Ӧ��ƽ������Ϊ �����¶��µ�ƽ�ⳣ��ΪK= ��
�ӻ�ѧƽ���ƶ��ĽǶȷ��������A���ʵ�ת���ʿ��Բ�ȡ�Ĵ�ʩ�� ��ѡ�������ĸ����
a����ʱ����������� b��ƽ����ټ���6molA���� c������ѹǿ d��ʹ�ô��� e��ƽ����ټ���2molC����
��1��N�ĵ���ʽΪ ��M�����к��� ������ԡ��Ǽ��ԡ�����
��2��D��E��1:1�γɵĻ�������ˮ��Ӧ�Ļ�ѧ��Ӧ����ʽ
(3)A��B��C��D���γ������Ӻ������Ӹ�������1��1�����ӻ�����X��A��D��E���γɻ�����Y��X��Y�����ʵ���֮��1��2���ȷ�Ӧ��д����Ӧ�Ļ�ѧ����ʽ
��Ӧ��ˮ��Һ�� ����ᡱ������С�����,ԭ���� �������ӷ���ʽ��ʾ��
��4����N��D�ĵ��ʡ�KOH��Һ����ԭ��أ����������C�ĵ��ʡ����为����ӦΪ ��һ��ʱ�����ҺpH ���������С�����䡱����
 ��5����һ���¶��£���4 mol C���ʺ�12 mol A����ͨ�뵽���Ϊ2L���ܱ������У�������Ӧ��2 min�ﵽƽ��״̬ʱ��A���ʵ�ת������50%������A���ʱ�ʾ�÷�Ӧ��ƽ������Ϊ �����¶��µ�ƽ�ⳣ��ΪK= ��
��5����һ���¶��£���4 mol C���ʺ�12 mol A����ͨ�뵽���Ϊ2L���ܱ������У�������Ӧ��2 min�ﵽƽ��״̬ʱ��A���ʵ�ת������50%������A���ʱ�ʾ�÷�Ӧ��ƽ������Ϊ �����¶��µ�ƽ�ⳣ��ΪK= ���ӻ�ѧƽ���ƶ��ĽǶȷ��������A���ʵ�ת���ʿ��Բ�ȡ�Ĵ�ʩ�� ��ѡ�������ĸ����
a����ʱ����������� b��ƽ����ټ���6molA���� c������ѹǿ d��ʹ�ô��� e��ƽ����ټ���2molC����
��18�֣�
��1��(2��) N�ĵ���ʽΪ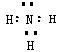 ����
����
��2����2�֣�2Na2O2 + 2CO2 ="=" 2Na2CO3 + O2 ��
��3����5�֣������𰸾����֣�NH4HCO3 + 2NaOH Na2CO3 + NH3 ��+ 2H2O ��
Na2CO3 + NH3 ��+ 2H2O ��
CO32- + H2O HCO3 - + OH
HCO3 - + OH
 ��4��(3��) 2 NH3��6e
��4��(3��) 2 NH3��6e +6 OH
+6 OH N2+6H2O (2��) ��С
N2+6H2O (2��) ��С
��5����6�֣�v(H2) ��1.5mol/L-1min-1�� K��4/7 a c e
��1��(2��) N�ĵ���ʽΪ
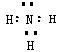 ����
������2����2�֣�2Na2O2 + 2CO2 ="=" 2Na2CO3 + O2 ��
��3����5�֣������𰸾����֣�NH4HCO3 + 2NaOH
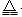 Na2CO3 + NH3 ��+ 2H2O ��
Na2CO3 + NH3 ��+ 2H2O ��CO32- + H2O
 HCO3 - + OH
HCO3 - + OH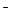
 ��4��(3��) 2 NH3��6e
��4��(3��) 2 NH3��6e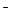 +6 OH
+6 OH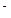 N2+6H2O (2��) ��С
N2+6H2O (2��) ��С��5����6�֣�v(H2) ��1.5mol/L-1min-1�� K��4/7 a c e
��

��ϰ��ϵ�д�
�����Ŀ

 ��B��ͬ���ڵ�һ��������С��Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2��
��B��ͬ���ڵ�һ��������С��Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2�� 1molF���뷴Ӧ��ʱ��ת�Ƶĵ�����ĿΪ ����Ӧ�����������뻹ԭ�������ʵ���֮��Ϊ ��������Dͨ��BaCl2��Һ�У�δ�����ǣ����Ҫʹ��Һ����ǣ�������Һ��ͨ�˵����������
1molF���뷴Ӧ��ʱ��ת�Ƶĵ�����ĿΪ ����Ӧ�����������뻹ԭ�������ʵ���֮��Ϊ ��������Dͨ��BaCl2��Һ�У�δ�����ǣ����Ҫʹ��Һ����ǣ�������Һ��ͨ�˵����������  (��2��)��
(��2��)�� ����Ӧ�۵����ӷ���ʽ�� ��2molG��lmolB��������2mol�����ʣ������ʵķ���������ԭ�Ӷ��ﵽ8�����ȶ��ṹ�������ʽΪ ��
����Ӧ�۵����ӷ���ʽ�� ��2molG��lmolB��������2mol�����ʣ������ʵķ���������ԭ�Ӷ��ﵽ8�����ȶ��ṹ�������ʽΪ ��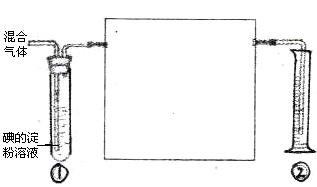
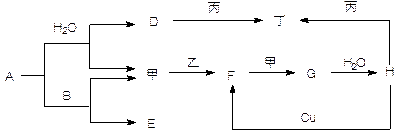
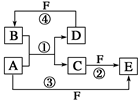
 ________________________________________��
________________________________________�� �ʣ��ҷ�Ӧ����ˮ��Һ�н��С�
�ʣ��ҷ�Ӧ����ˮ��Һ�н��С� �����ֳ���Ԫ��W��X��Y��Z�������γ�һ�ֻ�������������W��Y���������ǵ����������Ҫ���ʣ�X��������ȿ��Ժ�ǿ�ᡢ����Ժ�ǿ�Ӧ��Z�����ש��ɫ�ͺ�ɫ����
�����ֳ���Ԫ��W��X��Y��Z�������γ�һ�ֻ�������������W��Y���������ǵ����������Ҫ���ʣ�X��������ȿ��Ժ�ǿ�ᡢ����Ժ�ǿ�Ӧ��Z�����ש��ɫ�ͺ�ɫ���� �������
������� ���õ���ʽ��ʾ����̬��
���õ���ʽ��ʾ����̬�� ���� ��
���� �� ��
��