��Ŀ����
(ÿ��1�� ��10��) A��B��C��D�����ֶ�����Ԫ�أ�E�ǹ���Ԫ�ء�A��B��Cͬ���ڣ�C��Dͬ���壬A��ԭ�ӽṹʾ��ͼΪ��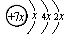 ��B��ͬ���ڵ�һ��������С��Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2��
��B��ͬ���ڵ�һ��������С��Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2��
�ش��������⣺
��1����Ԫ�ط��ű�ʾD��������(��ϡ������Ԫ����)��һ����������Ԫ����__________���縺������Ԫ����__________��
��2�� D���⻯���C���⻯��ķе�_____����"��"��"��"����ԭ��____________��
��3��EԪ�������ڱ���λ���� ��
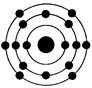
��4������D�ĺ�������Ų�ͼ_________________________________________�������Ų���ѭ�� ԭ����____________ԭ����____________����
��5���õ���ʽ��ʾB��������γɹ��̣�______________________________��
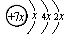 ��B��ͬ���ڵ�һ��������С��Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2��
��B��ͬ���ڵ�һ��������С��Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺
��1����Ԫ�ط��ű�ʾD��������(��ϡ������Ԫ����)��һ����������Ԫ����__________���縺������Ԫ����__________��
��2�� D���⻯���C���⻯��ķе�_____����"��"��"��"����ԭ��____________��
��3��EԪ�������ڱ���λ���� ��
��4������D�ĺ�������Ų�ͼ_________________________________________�������Ų���ѭ�� ԭ����____________ԭ����____________����
��5���õ���ʽ��ʾB��������γɹ��̣�______________________________��
(ÿ��1�֣���10��) ��1��F F (2) �� ��ΪNH3���Ӽ��γ����
(3)�����ڵ��Ģ��� (4)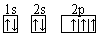 ��������� ���� ����
��������� ���� ����
(5)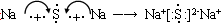
(3)�����ڵ��Ģ��� (4)
 ��������� ���� ����
��������� ���� ����(5)
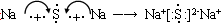
����Ԫ�����ڱ��Ľṹ��Ԫ�������ɵ�Ӧ�õȡ�
����Ԫ�صĽṹ���й����ʿ�֪��A��B��C��D��E�ֱ���Si��Na��P��N��Fe��
��1����Ԫ���ǵڶ�����Ԫ�أ����ڷǽ�����Խǿ����һ������Խ��F����ǿ�ķǽ���Ԫ�أ����Ե�һ������������F��ͬ���ǽ�����Խǿ���縺��Ҳ�����ģ����Ҳ��F��
��2������NH3���Ӽ��γ�������������ķе����PH3�ġ�
��3������ԭ��������26��λ�ڵ������ڵڢ��塣
��4������ԭ�Ӻ�����ӵ��Ų����ɡ����ݹ���ԭ����֪����ԭ�ӵĺ�������Ų�ͼ
 ����Ϊ���������Ų���ѭ���������ԭ��������ԭ���ͺ��ع���
����Ϊ���������Ų���ѭ���������ԭ��������ԭ���ͺ��ع���
��5���ƺ�S�γɵ������ӻ�������γɹ�����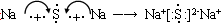 ��
��
����Ԫ�صĽṹ���й����ʿ�֪��A��B��C��D��E�ֱ���Si��Na��P��N��Fe��
��1����Ԫ���ǵڶ�����Ԫ�أ����ڷǽ�����Խǿ����һ������Խ��F����ǿ�ķǽ���Ԫ�أ����Ե�һ������������F��ͬ���ǽ�����Խǿ���縺��Ҳ�����ģ����Ҳ��F��
��2������NH3���Ӽ��γ�������������ķе����PH3�ġ�
��3������ԭ��������26��λ�ڵ������ڵڢ��塣
��4������ԭ�Ӻ�����ӵ��Ų����ɡ����ݹ���ԭ����֪����ԭ�ӵĺ�������Ų�ͼ
 ����Ϊ���������Ų���ѭ���������ԭ��������ԭ���ͺ��ع���
����Ϊ���������Ų���ѭ���������ԭ��������ԭ���ͺ��ع�����5���ƺ�S�γɵ������ӻ�������γɹ�����
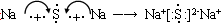 ��
��
��ϰ��ϵ�д�
�����Ŀ

 ��5����һ���¶��£���4 mol C���ʺ�12 mol A����ͨ�뵽���Ϊ2L���ܱ������У�������Ӧ��2 min�ﵽƽ��״̬ʱ��A���ʵ�ת������50%������A���ʱ�ʾ�÷�Ӧ��ƽ������Ϊ �����¶��µ�ƽ�ⳣ��ΪK= ��
��5����һ���¶��£���4 mol C���ʺ�12 mol A����ͨ�뵽���Ϊ2L���ܱ������У�������Ӧ��2 min�ﵽƽ��״̬ʱ��A���ʵ�ת������50%������A���ʱ�ʾ�÷�Ӧ��ƽ������Ϊ �����¶��µ�ƽ�ⳣ��ΪK= ��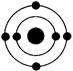


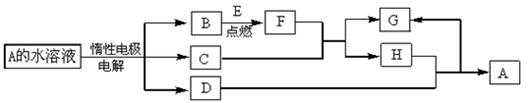

 B��C��D��ԭ��������С��18��A��Dͬ���壬B��C��ͬһ���ڣ�A��Dԭ�ӵ���������������1��Cԭ��������������Bԭ����2������C�����������Ǵ�����������2����A��B�����ڳ����¾�Ϊ���壬�����ڸ������������2��1��ȫ��Ӧ���������ڳ�������Һ�塣��Һ����D�����ܼ��ҷ�Ӧ����A�ĵ��ʡ�������Һ�����̪�Ժ�ɫ��ͬʱ��Һ�к�������ԭ�ӵĵ��Ӳ�ṹ��ͬ�������ӡ��ش��������⣺
B��C��D��ԭ��������С��18��A��Dͬ���壬B��C��ͬһ���ڣ�A��Dԭ�ӵ���������������1��Cԭ��������������Bԭ����2������C�����������Ǵ�����������2����A��B�����ڳ����¾�Ϊ���壬�����ڸ������������2��1��ȫ��Ӧ���������ڳ�������Һ�塣��Һ����D�����ܼ��ҷ�Ӧ����A�ĵ��ʡ�������Һ�����̪�Ժ�ɫ��ͬʱ��Һ�к�������ԭ�ӵĵ��Ӳ�ṹ��ͬ�������ӡ��ش��������⣺