��Ŀ����
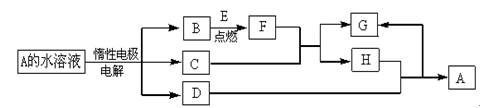
��ͼΪ����A��I��ת����ϵ�����ַ�Ӧ�������û���г���������BΪij���������Ҫ�ɷ֣�����һϵ�з�Ӧ�ɵõ�E��F��D��E������Ϊ���壬D��FΪ�������ʣ�����F����H��Ũ��Һ����ʱ������Ӧ��
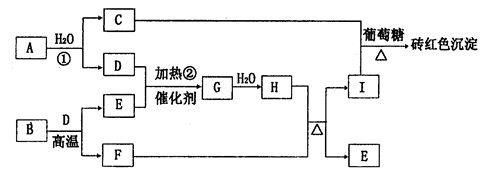
��ش��������⣺
��1��д��G�Ļ�ѧʽ��________________��
��2����Ӧ�ٵ����ӷ���ʽΪ__________________��
��3������F��H��Ũ��Һ������Ӧ�Ļ�ѧ����ʽΪ______________________��
��4����Pt���缫���I��Һ����ȫ����ĵ������Һ��_______________���������ĵ缫��ӦʽΪ____________________��
��5����֪ÿ16gE��D��ȫ��Ӧ���ų�24��75 kJ��������Ӧ�ڵ��Ȼ�ѧ����ʽΪ
________________________________________________��
��6����ҵ�Ʊ�Gʱ���������������Eռ7����Dռ11��������100������������뷴Ӧװ�ã�����������ָ���ԭ�¶Ⱥ�ѹǿ���Ϊ97��2�������E��ת����Ϊ________��

��ش��������⣺
��1��д��G�Ļ�ѧʽ��________________��
��2����Ӧ�ٵ����ӷ���ʽΪ__________________��
��3������F��H��Ũ��Һ������Ӧ�Ļ�ѧ����ʽΪ______________________��
��4����Pt���缫���I��Һ����ȫ����ĵ������Һ��_______________���������ĵ缫��ӦʽΪ____________________��
��5����֪ÿ16gE��D��ȫ��Ӧ���ų�24��75 kJ��������Ӧ�ڵ��Ȼ�ѧ����ʽΪ
________________________________________________��
��6����ҵ�Ʊ�Gʱ���������������Eռ7����Dռ11��������100������������뷴Ӧװ�ã�����������ָ���ԭ�¶Ⱥ�ѹǿ���Ϊ97��2�������E��ת����Ϊ________��
��1�� SO3 ��2�֣�
��2�� 2Na2O2+2H2O�T4Na+ + 4OH- + O2�� ��2�֣�
��3�� Cu + 2H2SO4 ��Ũ��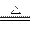 CuSO4 + SO2�� + 2H2O ��2�֣�
CuSO4 + SO2�� + 2H2O ��2�֣�
��4�� H2SO4 ��2�֣� 4OH����2H2O + O2��+4e�� ��2�֣�
��5��2SO2��2�֣�+ O2��g�� 2SO3��2�֣� ��H=" -198" kJ��mol-1 ��2�֣�
2SO3��2�֣� ��H=" -198" kJ��mol-1 ��2�֣�
��6��80% ��2�֣�
��2�� 2Na2O2+2H2O�T4Na+ + 4OH- + O2�� ��2�֣�
��3�� Cu + 2H2SO4 ��Ũ��
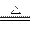 CuSO4 + SO2�� + 2H2O ��2�֣�
CuSO4 + SO2�� + 2H2O ��2�֣���4�� H2SO4 ��2�֣� 4OH����2H2O + O2��+4e�� ��2�֣�
��5��2SO2��2�֣�+ O2��g��
 2SO3��2�֣� ��H=" -198" kJ��mol-1 ��2�֣�
2SO3��2�֣� ��H=" -198" kJ��mol-1 ��2�֣���6��80% ��2�֣�
��

��ϰ��ϵ�д�
�����Ŀ

 R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�����ұ���JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�ء�
R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�����ұ���JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�ء�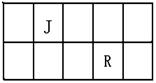
 ��
�� ��ʪ�Ŀ�����ð��ɫ��������Ӧ�Ļ�ѧ����ʽΪ___ __��
��ʪ�Ŀ�����ð��ɫ��������Ӧ�Ļ�ѧ����ʽΪ___ __��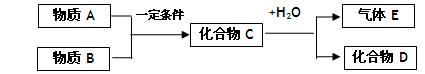
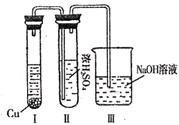
 ��5����һ���¶��£���4 mol C���ʺ�12 mol A����ͨ�뵽���Ϊ2L���ܱ������У�������Ӧ��2 min�ﵽƽ��״̬ʱ��A���ʵ�ת������50%������A���ʱ�ʾ�÷�Ӧ��ƽ������Ϊ �����¶��µ�ƽ�ⳣ��ΪK= ��
��5����һ���¶��£���4 mol C���ʺ�12 mol A����ͨ�뵽���Ϊ2L���ܱ������У�������Ӧ��2 min�ﵽƽ��״̬ʱ��A���ʵ�ת������50%������A���ʱ�ʾ�÷�Ӧ��ƽ������Ϊ �����¶��µ�ƽ�ⳣ��ΪK= ��