��Ŀ����
����Ŀ���ס�����λͬѧ���ʵ��ȷ��ij��HA��������ʣ�ʵ�鷽�����£�

�ף�ȡ������ͬ����������С��ȵ�п������֧�Թ��У�ͬʱ����Ũ�Ⱦ�Ϊ0.1 mol��L-1 ��HA��Һ��ϡ�����10 mL����ͼװ�ã��۲�����
�ң�����һ����pH�ƲⶨŨ��Ϊ 0.1 mol��L-1HA��Һ��pH��
��������ȡpH=3��HA��Һ5 mLϡ����500 mL������pH�Ʋ���pH��
�ش��������⣺
��1����ͬѧ��Ƶķ����У�˵��HA��������ʵ�ʵ��������___________ (�����)��
A. ��������ϡ��������Թ��Ϸ�������ͬʱ������һ����
B. ����HA��Һ���Թ��Ϸ������������
C. ����ϡ������Թ��Ϸ������������
��2����ͬѧ��Ƶķ���һ��˵��HA��������ʵ������ǣ�__________________
��3����ͬѧ��Ƶķ�������˵��HA��������ʵ�pH�ķ�ΧΪ__________________
��4����ͬѧΪ�˽�һ��֤�����������������ʵ���ƽ���ƶ���������������ʵ�飺
��ʹHA�ĵ���̶Ⱥ�c(H+)����С��c(A-)������ 0.1mol��L-1 ��HA��Һ�У�ѡ�����____________�Լ���
��ʹHA�ĵ���̶ȼ�С��c(H+)��c(A-)�������� 0.1mol��L-1 ��HA��Һ�У�ѡ�����_____________�Լ���
��5����������ˮ��ԭ���Ƕ����һ���������Ƚ������еķ���(ҩƷ����ȡ) ��֤��HA��������ʣ������Ʒ�����________________________________��
���𰸡�B ��� 0.1 mol��L-1 ��HA��Һ��pH > 1 3<PH<5 NaA���壨�����������𰸣� ���ʵ���Ũ�ȴ���0.1 mol��L-1��HA��Һ �ڳ����²�NaA��ҺpH����pH��7����֤��HA���������
��������
����������ʵĵ���ƽ���Ӱ�����ط���������ȫ���룬����������Ũ�Ȼ�С�����Ũ�ȣ������Ӧ���Σ�������ĵ��룬������Ũ�ȼ�С������ϴ�Ũ�ȵĶ�Ӧ���ᣬ����̶ȼ�С���������ӻ��������Ũ�Ȼ�����
(1) ����Ƶķ���������Ϊǿ�ᣬ��ȫ������HAΪ���ᣬ�ֵ��룬�������������Ũ�Ƚ�С����Ӧ���ʼ������Թ��Ϸ����������������B��ȷ��
��2�� ��� 0.1 mol��L-1 ��HA��Һ��pH > 1��˵����Һ�е�������Ũ��С��0.1mol/L��˵�����ȫ���룬Ϊ������ʣ�
(3) ȡpH=3��HA��Һ5 mLϡ����500 mL����Һ��HA�������������Ũ����ԭ����1/100������Ϊ������ʣ����ܼ������룬����������Ũ�ȴ���10-5mol/L���� 3<pH<5��
(4) ��HA ![]() A-+H+������ƽ���ƶ�ԭ��������ʹHA�ĵ���̶Ⱥ�c(H+)����С��c(A-)��������Ӧ���뺬��A-�����ʣ����� 0.1mol��L-1 ��HA��Һ��NaA���壻
A-+H+������ƽ���ƶ�ԭ��������ʹHA�ĵ���̶Ⱥ�c(H+)����С��c(A-)��������Ӧ���뺬��A-�����ʣ����� 0.1mol��L-1 ��HA��Һ��NaA���壻
��HA��Ũ������HA�ĵ���̶ȼ�С��c(H+)��c(A-)���������Կ�����ԭ��Һ�м������ʵ���Ũ�ȴ���0.1 mol��L-1��HA��Һ��
(5)HA��������ʣ����Ӧ��ǿ�����ܷ���ˮ����Լ��� ���Կ����ڳ����²�NaA��ҺpH����pH��7����֤��HA��������ʡ�

 �ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�����Ŀ���Ͼ�Ӳ�ʺϽ��к�̼����(WC)��������(Co)�����������������õ�ⷨ����WC���Ʊ�Co2O3�Ĺ������̼�ͼ���£�
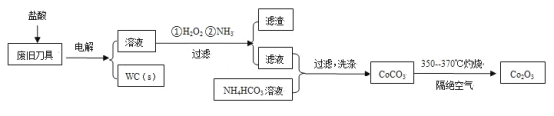
��֪�������������У����ֽ��������γ��������������pH��Χ���£�
�������� | Fe3+ | Fe2+ | Co2+ |
��ʼ������pH | 1.9 | 7.0 | 6.5 |
������ȫ��pH | 3.2 | 9.0 | 9.4 |
�ش��������⣺
��1���ԷϾɵ����������������������������Ϊ�������Һ�����ʱ�����ĵ缫��Ӧ�У�Co-2e-��Co2+��______��
��2��ͨ�백����Ŀ���ǵ�����Һ��pH����ȥ��Ԫ�ء��ɱ��е����ݿ�֪�������Ͽ�ѡ���pH�ķ�Χ��_______��
��3������CoCO3�����ӷ���ʽ��________��
��4��ʵ����NH4HCO3��Һ�Լ��ԡ��Ʊ�CoCO3ʱ�����ܽ���Һ����NH4HCO3��Һ�У�ԭ����_______��
��5����֪��Ksp(CoCO3)��1.4��10��13��Ksp(CoC2O4)��6.3��10��8�������ӳ���ת���Ƕȿ��ǣ���0.01mol/L Na2C2O4��Һ�м���CoCO3�����ܷ�ת��ΪCoC2O4������ͨ������˵����_________��
��6��ϴ��CoCO3����ֶ����ղ�Ʒ���Ȳ���Ӱ�죬���ڱ���ʱ����ɻ�����Ⱦ����Ҫԭ����____��
��7��CoCO3����Co2O3�Ļ�ѧ����ʽ��_________��





