��Ŀ����
4����1��18Oԭ�ӽṹʾ��ͼ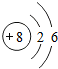 ��
����2��д�����л�ѧ����ʽ��
�ٹ�������������������ȼ��2Fe+3Cl2��������$\frac{\underline{\;��ȼ\;}}{\;}$2FeCl3
�ڣ�SCN��2 ������±�ء���±�أ���������Cl2��±�ص������ƣ���SCN��2�� NaOH��Һ��Ӧ��SCN��2+2NaOH=NaSCN+NaSCNO+H2O
��3���������������ԭ��Ӧ�Ļ�ѧ����ʽ����ƽ����
5K2C2O4+2KMnO4+8H2SO4=10CO2+2MnSO4+6K2SO4+8H2O��
���� ��1��18O��������Ϊ8��������Ϊ18��������=������=8��
��2��������������Ӧ�����Ȼ������ڸ����������������Ƶķ�Ӧ��д��
��3�������������Ԫ�ػ��ϼ۽���7-2=5�ۣ�̼Ԫ�ػ��ϼ�����4-0=4�ۣ�
��� �⣺��1��18O��������Ϊ8��������Ϊ18��������=������=8��ԭ�ӵĽṹʾ��ͼΪ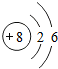 ���ʴ�Ϊ��
���ʴ�Ϊ��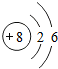 ��
��
��2��������������Ӧ�����Ȼ������뷴Ӧ�������أ�2Fe+3Cl2��������$\frac{\underline{\;��ȼ\;}}{\;}$2FeCl3���ʴ�Ϊ��2Fe+3Cl2��������$\frac{\underline{\;��ȼ\;}}{\;}$2FeCl3���ڸ����������������Ƶķ�Ӧ��֪��SCN��2+2NaOH=NaSCN+NaSCNO+H2O���ʴ�Ϊ����SCN��2+2NaOH=NaSCN+NaSCNO+H2O��
��3�������������Ԫ�ػ��ϼ۽���7-2=5�ۣ�̼Ԫ�ػ��ϼ�����4-0=4�ۣ����ϼ�������=���ϼ۽�������5K2C2O4+2KMnO4+8H2SO4�Tl0CO2��+2MnSO4+6K2SO4+8H2O���ʴ�Ϊ��5��2��8��10��2��6��8��
���� ���⿼��������ԭ��Ӧ��ƽ��Ϊ��Ƶ���㣬���շ�Ӧ��Ԫ�صĻ��ϼ۱仯��ԭ���غ���ƽΪ���Ĺؼ���ע�ط�����Ӧ�������Ŀ��飬��Ŀ�ѶȲ���

| A�� | �����Ͷ���������ʹƷ����Һ��ɫ | |
| B�� | �����������ϩ����ʹ��ˮ��Һ��ɫ | |
| C�� | �����������ϩ����ʹ���Ը��������Һ��ɫ | |
| D�� | ����Na2SO4�ͼ�ȩ����ʹ�����ʴ���Һ������ |
| A�� | 100mL 1mol•L-1NaCl ��Һ | B�� | 50mL 1mol•L-1AlCl3 ��Һ | ||
| C�� | 75mL 1mol•L-1BaCl2 ��Һ | D�� | 75mL 2mol•L-1NaCl��Һ |
2H2��g��+O2��g��=2H2O��g����H1=-483.6kJ•mol-1
2H2��g��+O2��g��=2H2O��l����H2=-571.6kJ•mol-1
�ݴ��жϣ�����˵����ȷ���ǣ�������
| A�� | H2��g����O2��g����Ӧ����H2O��g�������ȷ�Ӧ | |
| B�� | 1 molH2O��g��ת���1 molH2O��l���ų�44.0kJ���� | |
| C�� | 1 molH2O��l��ת���1 molH2O��g���ų�44.0kJ���� | |
| D�� | 1 molH2O��g��ת���1 molH2O��l���ų�88.0kJ���� |
| A�� | ������ | B�� | 75%�ľƾ���Һ | C�� | Fe��OH��3���� | D�� | ���� |
