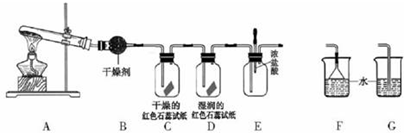
��Ŀ����
14�� ʵ������Ҫ0.1mol•L-1 NaOH��Һ450mL��0.5mol•L-1������Һ500mL��������������Һ����������ش��������⣺
ʵ������Ҫ0.1mol•L-1 NaOH��Һ450mL��0.5mol•L-1������Һ500mL��������������Һ����������ش��������⣺��1����ͼ��ʾ��������������Һ�϶�����Ҫ����AC������ţ�������������Һ�����õ��IJ����������ձ��������������������ƣ���
��2��������NaOH��Һʱ��
�ٸ��ݼ�����������ƽ��ȡNaOH������Ϊ2.0g��
����NaOH��Һ��ת��������ƿʱ����������������������ҺŨ�ȣ������������������=����0.1mol•L-1��
����NaOH�����ܽ��������������ƿ��ϴ�ձ���ϴ��Һ��������ƿ�����ݣ���������ҺŨ�ȣ������������������=����0.1mol•L-1��
��3��������������Һʱ��
��������������Ϊ98%���ܶ�Ϊ1.84g•cm-3��Ũ��������Ϊ13.6������������һλС����mL��
�����ʵ������15mL��20mL��50mL��Ͳ��Ӧѡ��15mL��Ͳ��ã�
�����ƹ������������ձ��н�Ũ�������ϡ�ͣ�ϡ��ʱ���������ǽ�Ũ���������ڻ�������ˮ�У����ò��������Ͻ��裮
���� ��1��������Һ��Ҫ���ֲ�����������Ͳ���ձ�������������ͷ�ιܡ�����ƿ��
��2��������450 mL������ƿ������NaOH��ҺҪ��500 mL������ƿ��m��NaOH��=c•V•M=0.1 mol•L-1��0.5 L��40 g•mol-1=2.0 g����NaOH����ˮ�ų������ȣ�Ӧ������ȴ�����º�����������ƿ�У������ݺ���Һ��ȴ�����º������С��Ũ��ƫ�ߣ�
��3����c��Ũ��•V��Ũ��=c��ϡ��•V��ϡ������$\frac{1000��1.84��98%}{98}$��V��Ũ��=0.5��0.5����V��Ũ����0.013 6 L=13.6 mL����ѡ��15 mL��Ͳ��ã����С����ע�����㣺����ˮ�������ڡ������裮
��� �⣺��1��������Һ��Ҫ���ֲ�����������Ͳ���ձ�������������ͷ�ιܡ�����ƿ���ʴ�Ϊ��AC���ձ�����������
��2��������450 mL������ƿ������NaOH��ҺҪ��500 mL������ƿ��m��NaOH��=c•V•M=0.1 mol•L-1��0.5 L��40 g•mol-1=2.0 g����NaOH����ˮ�ų������ȣ�Ӧ������ȴ�����º�����������ƿ�У������ݺ���Һ��ȴ�����º������С��Ũ��ƫ�ߣ�
�ʴ�Ϊ��2.0����������
��3����c��Ũ��•V��Ũ��=c��ϡ��•V��ϡ������$\frac{1000��1.84��98%}{98}$��V��Ũ��=0.5��0.5����V��Ũ����0.013 6 L=13.6 mL����ѡ��15 mL��Ͳ��ã����С����ע�����㣺����ˮ�������ڡ������裻
�ʴ�Ϊ��13.6��15����Ũ���������ڻ�������ˮ�У����ò��������Ͻ��裮
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ��Ƚϻ�����ע���c=$\frac{n}{V}$��������ԭ���������������ƹ���

 Сѧ��10���ӿ������100��ϵ�д�
Сѧ��10���ӿ������100��ϵ�д�| A�� | ���ô��⻯����ȥ�����е�������ϩ | |
| B�� | ����Һ�巴Ӧ������H2��Ӧ����ͬһ���͵ķ�Ӧ | |
| C�� | ������ʹKMnO4��Һ��ɫ�����Ա����ܷ���������Ӧ | |
| D�� | ��Ȳ���Է���ȡ����Ӧ���ӳɷ�Ӧ���Ӿ۷�Ӧ |
| A�� | ��Ӧ��ϵ����ѹ�㶨 | B�� | A��B��C��D�����ʵ���֮��Ϊ1��3��2��2 | ||
| C�� | c��A����c��B��=1��3 | D�� | 2V��B����=3V��C���� |
| A�� | C18H36O2 | B�� | C16H34O2 | C�� | C18H34O2 | D�� | C16H30O2 |
| X | Y | |
| Z | W |
��1��Wλ�����ڱ��е������ڣ��ڢ�A�壮
��2��X������Թ���+1�������ӣ������ʽ��
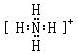 ��Y����̬�⻯����ȶ��Ա�Z����̬�⻯����ȶ���ǿ���ǿ��������������
��Y����̬�⻯����ȶ��Ա�Z����̬�⻯����ȶ���ǿ���ǿ����������������3��X������������ˮ���������⻯���ܻ�������M��M��ˮ��Һ�����Ե�ԭ����NH4++H2O?NH3•H2O+H+�������ӷ���ʽ��ʾ����
��4����Y��Z�����һ����̬������Q��Q����W�ĵ����ڳ�ʪ�����з�Ӧ����Ӧ�Ļ�ѧ����ʽ��SO2+Cl2+2H2O=2HCl+H2SO4��
����һ�������£�������Q��Y�ĵ��ʷ�Ӧ��ƽ��ʱ��������̬���ʣ���Ӧʱ��ÿת��4mol���ӷ���190.0kJ���÷�Ӧ���Ȼ�ѧ����ʽ��2SO2 ��g��+O2��g��?2SO3��g����H=-190.0 kJ•mol-1��
������⣺Na2S2O3��Na2SO4�ṹ���ƣ���ѧ�����Ƿ�Ҳ�����أ�
ʵ��̽����ȡ����Na2S2O3���壬����ˮ���Ƴ�Na2S2O3��Һ����������̽����
| ʵ����� | ʵ������ | ������ͣ������ӷ���ʽ��ʾ�� | |
| ̽���� | A���ò�����պȡNa2S2O3��Һ����pH��ֽ�в�������ֽ��ɫ�����ɫ������ | a����ҺpH=8 | i��S2O32-+H2O?HS2O3-+OH- |
| B����pH=2�������еμ�Na2S2O3��Һ | b���е���ɫ�����������ɫ���ǣ�����ɫ�̼�����ζ������� | ii��S2O32һ+2H+=S��+SO2�� +H2O | |
| ̽���� | C����������ˮ��pH��2���еμ�����Na2S2O3��Һ | c����ˮ��ɫ��dz | iii��S2O32-+4Cl2+5H2O=2SO42-+8Cl-+10H+ |
̽���٣�Na2S2O3�ʼ��ԣ�����ǿ�ᷴӦ��̽���ڣ����л�ԭ�ԣ�
�������ۣ���ͬѧ��̽���ڡ���Ӧ�����Һ�еμ���������Һ���۲쵽�а�ɫ�������������ݴ���Ϊ��ˮ�ɽ�Na2S2O3����������Ϊ�÷�������ȷ�����ȷ��������ȷ����������������ˮ��������ˮ��ͬ������Cl-��
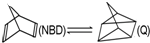 ��H=+88.62kJ•mol-1������˵������ȷ���ǣ�������
��H=+88.62kJ•mol-1������˵������ȷ���ǣ�������| A�� | 92gNBD��̫���������ȫת��ΪQʱ������88.62kJ���� | |
| B�� | NBD�ɿ���Ϊ���ܲ��� | |
| C�� | NBD��Q���ױ�����Ϊͬϵ�� | |
| D�� | NBD��Q���ױ�������ͬ���칹�� |