��Ŀ����
10������ʱ��8��12����23ʱ30�����ң������������������������ұ�ը���������α�ը�����Լ30�룬�ڶ��α�ը�������ߣ��൱��21��TNT����46öս��ʽѲ�����������µ����������������˾������ʾ���ù�˾�ִ�ҵ�����Ʒ����У�ѹ�������Һ�����壨�����ѹ����Ȼ���ȣ�����ȼҺ�壨����ͪ�����������ȣ�����ȼ���塢��ȼ��Ʒ����ʪ��ȼ��Ʒ�������ơ�����þ����ǡ�������ά�ء���ʯ����ƺϽ�ȣ������������л������������ء������Ƶȣ�������Ʒ���軯�ơ��ױ������������ȣ�����ʴƷ�����ࣨ���ᡢ���ᡢ�����ᡢ�ռ��ȣ��ȶ�����࣮��֪���軯�⣨HCN����״̬��ΪҺ�壮�軯�����ڿ����о�����ɢ���ڿ����п�ȼ�գ��軯���ڿ����еĺ����ﵽ5.6%��12.8%ʱ�����б�ը�ԣ����������ھ綾�࣮�����ᣨHCN����̼���������µĵ��볣���ֱ�Ϊ��HCN��Ka=6.2��10-10mol•L-1��H2CO3��Ka1=4.4��10-7mol•L-1��Ka2=4.7��10-11mol•L-1
���û�ѧ����ʽ�ش����и��⣺
��1��������������ȼ�������£�44g����������ȫȼ�շų� 1122.1kJ��������д����������ȼ���ȵ��Ȼ�ѧ��Ӧ����ʽ��CH3COOCH2CH3��l��+5O2��g��=4CO2��g��+4H2O��l������H=-2244.2kJ/mol
��2����ȼ�������ƣ�����ȼ���Ż�ʱ���ֽܷ����һ�������������ƣ����û�ѧ����ʽ��ʾԭ��4NaNO3=4NaNO2+O2��
��3����ȼ��������ƣ���ֹ��ˮǹ��ˮ������û�ѧ����ʽ��ʾԭ��2Na+2H2O=2NaOH+H2��
��4���������Ʒ�������ʱ����ֹ����ĭ������𣬷������ױ����������ɣ����û�ѧ����ʽ��ʾԭ��NaCN+CO2+H2O=HCN+NaHCO3��
��5�����������������ڡ����衱���軯�ƣ���Ӧ�����������ʣ���д���÷�Ӧ�Ļ�ѧ����ʽ��5NaClO+2NaCN=5NaCl+CO2��+N2��+Na2CO3
��5NaClO+2NaCN+H2O=5NaCl+N2��+2NaHCO3��
���� ��1������ȼ���ȵĸ�����Ȼ�ѧ����ʽ����д����д����ע�����ʵľۼ�״̬���ʱ�ļ��㣻
��2����ȼ�������ƣ�����ȼ���Ż�ʱ���ֽܷ����һ�������������ƣ�������Ϊ������
��3���ƺ�ˮ��Ӧ���ɾ��п�ȼ�Ե�������ͬʱ�����������ƣ�
��4����ĭ����������ɶ�����̼��������̼�ܺ������Ʒ�Ӧ�����ж����壻
��5���������ƺ������Ʒ���������ԭ��Ӧ�����Ȼ��ơ�������̼�����ƻ������̼��̼���ƣ�
��� �⣺��1��44g�������������ʵ���Ϊ$\frac{44g}{88g/mol}$=0.5mol����ȫȼ�ղ����ɶ�����̼��Һ̬ˮʱ���ų�����Ϊ1122.1kJ��ȼ������1mol��ȼ����ȫȼ�������ȶ�������ʱ�ų�����������Ӧ���Ȼ�ѧ����ʽ����ȼ����дΪ��CH3COOCH2CH3��l��+5O2��g��=4CO2��g��+4H2O��l������H=-2244.2kJ/mol��
�ʴ�Ϊ��CH3COOCH2CH3��l��+5O2��g��=4CO2��g��+4H2O��l������H=-2244.2kJ/mol��
��2����ȼ�������ƣ�����ȼ���Ż�ʱ���ֽܷ������������������ƣ���ӦΪ��4NaNO3=4NaNO2+O2����
�ʴ�Ϊ��4NaNO3=4NaNO2+O2����
��3����ȼ��������ƣ���ֹ��ˮǹ��ˮ�������Ϊ�ƺ�ˮ��Ӧ�����������ƺ��������������п�ȼ�ԣ���ӦΪ��2Na+2H2O=2NaOH+H2����
�ʴ�Ϊ��2Na+2H2O=2NaOH+H2����
��4����ĭ��������ԭ����ʹ����ĭ��������ʱ�������������������̼����ĭ��������ճ���ڿ�ȼ���ϣ�ʹ��ȼ��������������ﵽ����Ŀ�ģ����������ܺͶ�����̼��Ӧ��NaCN+CO2+H2O=HCN+NaHCO3���軯�⣮����Ҳ���ھ綾���ʣ����ڿ�������ɢ�����´����ֺ��������������Ʒ�������ʱ����ֹ����ĭ�������
�ʴ�Ϊ��NaCN+CO2+H2O=HCN+NaHCO3��
��5�����������������ڡ����衱���軯�ƣ���Ӧ������Ϊ�������ƺ������Ʒ���������ԭ��Ӧ��5NaClO+2NaCN=5NaCl+CO2��+N2��+Na2CO3��5NaClO+2NaCN+H2O=5NaCl+N2��+2NaHCO3�������������ʣ�
�ʴ�Ϊ��5NaClO+2NaCN=5NaCl+CO2��+N2��+Na2CO3��5NaClO+2NaCN+H2O=5NaCl+N2��+2NaHCO3��
���� ���⿼�黯ѧ��Ӧ����ʽ����д�����շ�Ӧԭ����ȼ���ȡ��Ȼ�ѧ����ʽ����д�ǽ��Ĺؼ�����Ŀ�Ѷ��еȣ�

| A�� | ��ϩ�붡ϩ | B�� | �����붡Ȳ | C�� | ��ϩ����Ȳ | D�� | �������ϩ |
| A�� | �����£�pH=3�ļ�����Һ��c��H+����pH=11�İ�ˮ��Һ����ˮ���������c��OH-����� | |
| B�� | �к� 0.1 mol•L-1 �������к� 0.01 mol•L-1�Ĵ�������ͬ�ּ���Һ�����ʵ�����ͬ | |
| C�� | ��NH4Cl��Һ����������NaOH���壬��Һ�ĵ�����������ǿ | |
| D�� | ��ʯ��ˮ�м�������CaO���ָ������º���Һ��c��OH-��������������ǿ |
| A�� | �մ���ϡ���ᷴӦ��HCO3-+H+�TCO2��+H2O | |
| B�� | Fe2O3��HI��Fe2O3+6H+�T2Fe3++3H2O | |
| C�� | ��NH4Al��SO4��2��Һ�еμ�Ba��OH��2 ��Һ��SO42-�պ���ȫ������NH4++Al3++2SO42-+2Ba2++4OH-�T2BaSO4��+NH3•H2O+Al��OH��3�� | |
| D�� | ��NaClO��Һ��ͨ������SO2��SO2+ClO-+H2O�TSO42-+Cl-+2H+ |
| A�� | ����N2ʹѹǿ����ɼӿ췴Ӧ���� | B�� | A��ƽ����Ӧ����Ϊ��0.4mol/��L•min�� | ||
| C�� | ƽ��״̬ʱB��C�ķ�Ӧ������� | D�� | C��ƽ����Ӧ����Ϊ��0.2mol/��L•min�� |
| A�� | 0.4mol/L | B�� | 0.2mol/L | C�� | 0.1mol/L | D�� | 0.08mol/L |
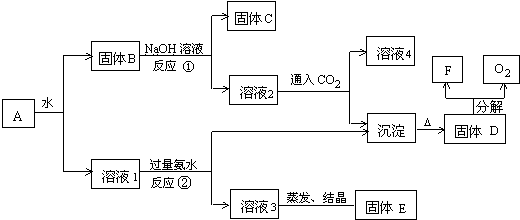
 ��������Ϊ������ȩ����
��������Ϊ������ȩ����
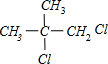 PMAA
PMAA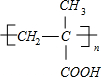
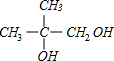 +O2$��_{��}^{Cu}$2
+O2$��_{��}^{Cu}$2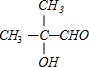 +2H2O��
+2H2O�� $��_{��}^{Ũ����}$CH2=C��CH3��-COOH+H2O��
$��_{��}^{Ũ����}$CH2=C��CH3��-COOH+H2O��