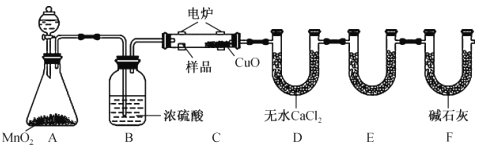
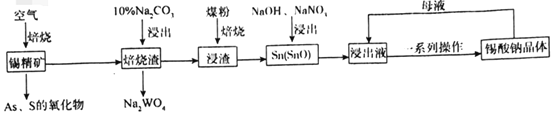
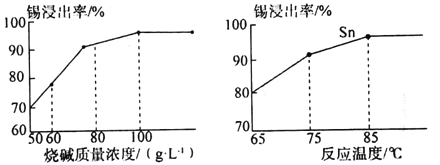
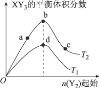
��Ŀ����
����Ŀ�������£��ֱ�ȡ10 mL pH��Ϊ2�����ᡢ������Һ�ֱ���10mL 0.01 mol L-1 NaHCO3��Һ��ϣ�ʵ���ò���CO2��������(V)��ʱ��(t)�ı仯��ͼ��ʾ������˵������ȷ����
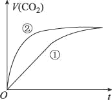
A.�ٱ�ʾ��������ķ�Ӧ����
B.��Ϻ����Һ�У�c(CH3COO��)>c(C1��)
C.�������ǰ������Һ��ˮ�ĵ���̶ȣ�NaHCO3��Һ>������Һ=����
D.�����NaHCO3��Һ��Ϻ����õ���Һ��:c(CH3COO��)+ c(CH3COOH) = 0.005 mol L-1
���𰸡�D
��������
A. pH��Ϊ2�����ᡢ������Һ˵����ʼH+����Ũ����ȣ���������Һ�д��ڵ���ƽ�⣬��������NaHCO3��Ӧʱ��H+����Ũ�ȼ�С��ƽ�������ƶ������ϲ���H+������������ȫ��������ᣬ������NaHCO3��Ӧ���ʻ���죬���ͼ�����б��С�ģ����ٱ�ʾ��������ķ�Ӧ���ߣ�A����ȷ��
B.���pH��Ϊ2�����ᡢ������Һ��֪c(HCl)=0.01mol/L����c(CH3COOH)>0.01mol/L���ֱ���0.01 mol L-1NaHCO3��Һ�������ϣ�������NaHCO3��Һǡ����ȫ��Ӧ���õ�������ΪNaCl����֪c(Na+)= c(Cl��)��������NaHCO3��Һ��Ӧ���õ���NaCl������CH3COONa������CH3COOH������CH3COOH�ĵ���̶ȴ���CH3COO����ˮ��̶ȣ����c(CH3COO��)> c(Na+)= c(Cl��)��B����ȷ��
C.���ᡢ������Һ��pH��Ϊ2��˵�����߶�ˮ��������Ƴ̶�һ������NaHCO3��Һ�Լ��ԣ�HCO3���ĵ���̶�������ˮ��̶ȣ����HCO3���ٽ�ˮ�ĵ��룬���������ǰ������Һ��ˮ�ĵ���̶ȣ�NaHCO3��Һ>������Һ=���ᣬC����ȷ��
D. c(CH3COOH)>0.01mol/L������������0.01 mol L-1 NaHCO3��Һ��ϣ���Ӧ����ʽΪCH3COOH+NaHCO3=CH3COONa+CO2��+H2O�����ݻ�ѧ��������c(CH3COONa)=![]() mol/L=0.005mol/L���Ҵ�����������������غ�֪c(CH3COO��)+ c(CH3COOH) > 0.005 mol L-1��D�����
mol/L=0.005mol/L���Ҵ�����������������غ�֪c(CH3COO��)+ c(CH3COOH) > 0.005 mol L-1��D�����
��ѡD��

 ��Уͨ��֤��Ч��ҵϵ�д�
��Уͨ��֤��Ч��ҵϵ�д�����Ŀ��2018�꣬�����˳��ˡ�����Э����ʵ���ٹ�ҵ��ս�ԣ����й�ȴ�Ӵ��˻������ȣ�����ڹ�����ҹ������εĴ���������ҹ�������ǿ��������CO2���⻯�ϳɼ״������Ĺ�ҵ�������о���ʵ�ֿɳ�����չ��
(1)��֪��I.CO2(g)+H2(g)![]() H2O(g)+CO(g) ��H1=+41.1kJ/mol
H2O(g)+CO(g) ��H1=+41.1kJ/mol
II.CO(g)+2H2(g)![]() CH3OH(g) ��H2=��90.0kJ/mol
CH3OH(g) ��H2=��90.0kJ/mol
д��CO2���⻯�ϳɼ״����Ȼ�ѧ����ʽ:________��
(2)Ϊ���CH3OH�IJ��ʣ�������Ӧ���õ�������________(��ѡ����ĸ����
a.���¸�ѹ b.���µ�ѹ c.���µ�ѹ d.���¸�ѹ
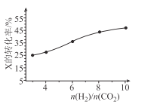
(3)250��ʱ���ں����ܱ���������CO2(g)���⻯�ϳ� CH3OH(g)����ͬͶ�ϱ�[n(H2)/n(CO2)]ʱij��Ӧ��X��ƽ��ת���ʱ仯������ͼ��ʾ����Ӧ��X��_______(����CO2������H2�������ж�������_______��
(4)250��ʱ�������Ϊ2.0 L�ĺ����ܱ������м���6mol H2��2mol CO2�ʹ������� CO2���⻯�ϳɼ״��ķ�Ӧ��10 minʱ��Ӧ�ﵽƽ�⣬���c(CH3OH) = 0.75 mol L-1��
��ǰ10 min�ڵ�ƽ����Ӧ����H2=_______ mol L-1 min-1��
�����¶��£��÷�Ӧ�Ļ�ѧƽ�ⳣ��K =_______��
�������ͷ�Ӧ�����뷴Ӧ��ת���ʺͲ����ѡ���Ը߶���ء�������ͬͶ�ϱȺ���ͬ��Ӧʱ�䣬����ʵ���������±���ʾ��
ʵ���� | �¶�(K) | ���� | CO2ת����(%) | �״�ѡ����(%) |
A | 543 | Cu/ZnO���װ� | 12.3 | 42.3 |
B | 543 | Cu/ZnO����Ƭ | 11.9 | 72.7 |
C | 553 | Cu/ZnO���װ� | 15.3 | 39.1 |
D | 553 | Cu/ZnO����Ƭ | 12.0 | 70.6 |
�����ϱ��������ݣ���CO2�����״�������ѡ��Ϊ________������ĸ����