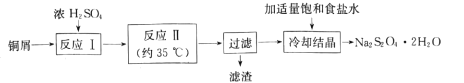
ЬтФПФкШн
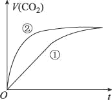
ЁОЬтФПЁПФГЛЏбЇаЁзщбаОПдкЦфЫћЬѕМўВЛБфЪБЃЌИФБфУмБеШнЦїжаФГвЛЬѕМўЖдX2 (g)+3Y2 (g)=2XY3(g)ЛЏбЇЦНКтзДЬЌЕФгАЯьЃЌЕУЕНШчЭМЫљЪОЕФЧњЯпЃЈЭМжаTБэЪОЮТЖШЃЌnБэЪОЮяжЪЕФСПЃЉЃЌЯТСаХаЖЯе§ШЗЕФЪЧ
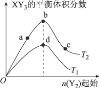
A.ШєT2>T1ЃЌдђе§ЗДгІвЛЖЈЪЧЗХШШЗДгІ
B.T2КЭn (X2)ВЛБфЃЌДяЕНЦНКтЪБЃЌXY3ЕФЮяжЪЕФСПЃКc>b>a
C.T2КЭn(X2)ВЛБфЃЌДяЕНЦНКтЪБЃЌX2ЕФзЊЛЏТЪ:b>a>c
D.ШєT2>T1ЃЌДяЕНЦНКтЪБbЁЂdЕуЕФе§ЗДгІЫйТЪ:v(d)>v (b)
ЁОД№АИЁПB
ЁОНтЮіЁП
XY3(g)ЕФЦНКтЬхЛ§ЗжЪ§ЕШгкXY3(g)ЕФЮяжЪЕФСПГ§вдX2 (g)ЁЂY2 (g)ЁЂXY3(g)ЕФЮяжЪЕФСПжЎКЭЃЌдіМгY2 (g)ЛсЪЙЕУЦНКте§ЯђвЦЖЏЃЌXY3(g)ЕФЮяжЪЕФСПБфДѓЃЌвђЖјXY3(g)ЕФЦНКтЬхЛ§ЗжЪ§ЯШдіДѓЃЌЕЋЫцзХY2 (g)БфЕУИќЖрЃЌX2 (g)ЁЂY2 (g)ЁЂXY3(g)ЕФЮяжЪЕФСПжЎКЭвВЛсБфЕУИќДѓЃЌXY3(g)ЕФЦНКтЬхЛ§ЗжЪ§ЗДЕЙМѕаЁЁЃ
A. Y2 (g)Ц№ЪМЭЖШыСПЯрЕШЪБЃЌШєT2>T1ЃЌЧвT2ЪБXY3(g)ЕФЦНКтЬхЛ§ЗжЪ§ДѓгкT1ЪБXY3(g)ЕФЦНКтЬхЛ§ЗжЪ§ЃЌЫЕУїЮТЖШдНИпЃЌЦНКте§ЯђвЦЖЏЃЌе§ЗДгІвЛЖЈЪЧЮќШШЗДгІЃЌAЯюДэЮѓЃЛ
B. T2КЭn (X2)ВЛБфЃЌдіМгY2 (g)ЛсЪЙЕУЦНКте§ЯђвЦЖЏЃЌXY3(g)ЕФЮяжЪЕФСПБфДѓЃЌМДДяЕНЦНКтЪБЃЌXY3ЕФЮяжЪЕФСПЃКc>b>aЃЌBЯюе§ШЗЃЛ
C. T2КЭn(X2)ВЛБфЃЌДяЕНЦНКтЪБЃЌдіМгY2 (g)ЛсЪЙЕУЦНКте§ЯђвЦЖЏЃЌX2 (g)ЮяжЪЕФСПвЛжБМѕаЁЃЌДяЕНЦНКтЪБЃЌX2ЕФзЊЛЏТЪж№НЅдіДѓЃЌМДc>b>aЃЌCЯюДэЮѓЃЛ
D.ЮТЖШдНИпЃЌЗДгІЫйТЪдНДѓЃЌШєT2>T1ЃЌДяЕНЦНКтЪБbЁЂdЕуЕФе§ЗДгІЫйТЪ:v(d)<v (b)ЃЌDЯюДэЮѓЃЛ
ЙЪбЁBЁЃ

 гХбЇУћЪІУћЬтЯЕСаД№АИ
гХбЇУћЪІУћЬтЯЕСаД№АИ