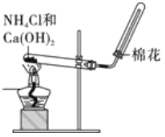
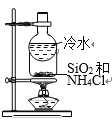
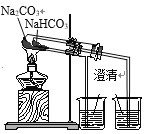
��Ŀ����
����Ŀ��ʵ������һ�ֹ�ҵ����![]() ��Ҫ�ɷ�Ϊ
��Ҫ�ɷ�Ϊ![]() ��
��![]() ������Fe��Cu��������
������Fe��Cu��������![]() Ϊԭ���Ʊ�
Ϊԭ���Ʊ�![]() ʵ��������£�
ʵ��������£�

![]() ���ܹ����У�
���ܹ����У�![]() �����Ļ�ѧ����ʽΪ______��
�����Ļ�ѧ����ʽΪ______��
![]() ����
����![]() ������Ϊ______��
������Ϊ______��
![]() ���й��ڷ�Һ©������ʹ�÷�������������ȷ����______
���й��ڷ�Һ©������ʹ�÷�������������ȷ����______![]() ��
��
A ʹ��ǰҪ�ȼ�鲣�������������Ƿ�©Һ
B �������л���ȡ����Ļ��Һת�Ƶ���Һ©���У����ϲ�������������ѹס��Һ©����������������ס��������ת©��������������ʱ������������
C ��Һʱ�����������Ͽڲ���������֤�ܷ⣬�Է�ֹ�л���ȡ���ӷ�
D ��ʵ���У���![]() ���л���һ�����¿ڷų���ˮ����Ͽڵ���
���л���һ�����¿ڷų���ˮ����Ͽڵ���
���𰸡�![]() ��
��![]() ����Ϊ
����Ϊ![]() �����ڳ��� AB
�����ڳ��� AB
��������
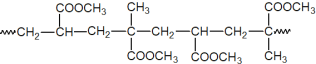
������Ҫ�ɷ�ΪMgCO3��Mg2SiO4������Fe��Cu�����������![]() ���������ܣ����ˣ���������ͭ�����ᣬ��Һ�к�������þ����������������H2O2�����������ӣ�Ȼ������л���ȡ����ȡFe3+����Һ��ɳ�ȥ��Һ�е�Fe3+����Һ��ˮ��Һ�к���Mg2+�����˺�����Һ�м���̼������Һ��������MgCO3���������յõ�MgCO33H2O�Դ˽����⡣
���������ܣ����ˣ���������ͭ�����ᣬ��Һ�к�������þ����������������H2O2�����������ӣ�Ȼ������л���ȡ����ȡFe3+����Һ��ɳ�ȥ��Һ�е�Fe3+����Һ��ˮ��Һ�к���Mg2+�����˺�����Һ�м���̼������Һ��������MgCO3���������յõ�MgCO33H2O�Դ˽����⡣
�����ܹ����У�Mg2SiO4�����Ļ�ѧ����ʽΪ![]() ��
��
�ʴ�Ϊ��![]() ��
��
�Ƽ���H2O2��Һ����������ӷ���������ԭ��Ӧ����Fe2+����ΪFe3+�����ڳ��ӣ�
�ʴ�Ϊ����Fe2+����ΪFe3+�����ڳ��ӣ�
��A.��Һ©������ʢװҺ�壬ʹ��ǰҪ�ȼ�鲣�������������Ƿ�©Һ����A��ȷ��
B.�������л���ȡ����Ļ��Һת�Ƶ���Һ©���У�Ӧ����ҡ�ȣ������ϲ�������������ѹס��Һ©����������������ס��������ת©��������������ʱ������������������ѹǿ����B��ȷ��
C.��Һʱ��Ӧ���Ͽڲ�������ʹҺ��˳������������C����
D.��֪���л����ܶ���ˮ���ܶȵĴ�С��ϵ����ȷ���л�������һ�㣬��D����
�ʴ�Ϊ��AB��

����Ŀ������ͼʾװ������ʾ��ʵ���У�û������������ԭ��Ӧ����
|
|
|
|
A��պ��Ũ�����Ũ��ˮ�IJ��������� | B����˿��������ͭ��Һ�� | C���ⶨ�������������� | D���������м���Ũ���� |
A.AB.BC.CD.D