��Ŀ����
15����ԭ��������С��20��A��B��C��D����Ԫ�أ���֪����A��B��ͬһ���壬C��D��ͬһ���ڣ�����Ԫ�������γ�A2C��A2C2��B2C2��DC2�Ȼ������B����������C�������Ӻ�����Ӳ�ṹ��ͬ����B2C2��A2C��DC2��Ӧ������C2���壻��B�ĵ�����A2C��Ӧ����A2���壬A2��C2�������ɷ�����ը�����ɳ�������ɫ����ζ��Һ��A2C���Իش���1��д��A��D��Ԫ�ص����ƣ�A�⡢D̼
��2������B���Ӻ�C���ӵĽṹʾ��ͼ
 ��
�� �����������У��뾶��С����Na+�������ӷ��ţ���
�����������У��뾶��С����Na+�������ӷ��ţ�����3��д��A2C2��B2C2�ĵ���ʽ��A2C2
 B2C2
B2C2 ��
����4��д��B2C2��DC2��Ӧ�Ļ�ѧ����ʽ2Na2O2+2CO2=2Na2CO2+O2��
���� ԭ��������С��20��A��B��C��D����Ԫ�أ�����A2������C2�������ɷ�����ը�����ɳ�������ɫ����ζ��Һ��A2C����AΪHԪ�ء�CΪOԪ�أ�����Һ��ΪH2O��A��B��ͬһ���壬B����������C�������Ӻ�����Ӳ�ṹ��ͬ��B�ĵ�����A2C��Ӧ����A2���壬��BΪNa��Na����Ԫ�ؿ����γ�Na2O2��C��D��ͬһ���ڣ�Na2O2��H2O��DC2��Ӧ������O2���壬��DC2ΪCO2����DΪ̼Ԫ�أ��ݴ˽��
��� �⣺ԭ��������С��20��A��B��C��D����Ԫ�أ�����A2������C2�������ɷ�����ը�����ɳ�������ɫ����ζ��Һ��A2C����AΪHԪ�ء�CΪOԪ�أ�����Һ��ΪH2O��A��B��ͬһ���壬B����������C�������Ӻ�����Ӳ�ṹ��ͬ��B�ĵ�����A2C��Ӧ����A2���壬��BΪNa��Na����Ԫ�ؿ����γ�Na2O2��C��D��ͬһ���ڣ�Na2O2��H2O��DC2��Ӧ������O2���壬��DC2ΪCO2����DΪ̼Ԫ�أ�
��1��������������֪��AΪ��Ԫ�ء�DΪ̼Ԫ�أ�
�ʴ�Ϊ���⣻̼��
��2��B����ΪNa+���ṹʾ��ͼΪ�� ��C����ΪO2-���ṹʾ��ͼΪ��
��C����ΪO2-���ṹʾ��ͼΪ�� �����Ӳ�ṹ��ͬ���˵����Խ�����Ӱ뾶ԽС�������Ӱ뾶��Na+��O2-��
�����Ӳ�ṹ��ͬ���˵����Խ�����Ӱ뾶ԽС�������Ӱ뾶��Na+��O2-��
�ʴ�Ϊ�� ��
�� ��Na+��
��Na+��
��3��H2O2�ĵ���ʽΪ ��Na2O2�ĵ���ʽΪ��
��Na2O2�ĵ���ʽΪ�� ��
��
�ʴ�Ϊ�� ��
�� ��
��
��4��B2C2��DC2��Ӧ�ǹ��������������̼��Ӧ����̼��������������Ӧ��ѧ����ʽΪ��2Na2O2+2CO2=2Na2CO2+O2��
�ʴ�Ϊ��2Na2O2+2CO2=2Na2CO2+O2��
���� ���⿼��Ԫ�ػ������ƶϣ��ƶ�ͻ�ƿ�Ϊ������A2������C2�������ɷ�����ը�����ɳ�������ɫ����ζ��Һ��A2C�����ٽ�����ʵ���ɼ�������Ӧ�ƶϣ����ضԻ�ѧ����Ŀ��飬�Ѷ��еȣ�

 ��У����ϵ�д�
��У����ϵ�д�| A�� | �����CO2��NO��SO2�� | B�� | �NaOH��KOH��Na2CO3 | ||
| C�� | ��Σ�NH4Cl��NH4NO3��NH3•H2O | D�� | ���������Na2O��CaO��Al2O3 |
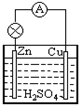
| A�� | Zn-2e-=Zn2+ | B�� | Cu-2e-=Cu2+ | C�� | 2H++2e-=H2�� | D�� | Cu2++2e-=Cu |
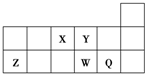
| �� ���� | IA | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 1 | a | |||||||
| 2 | b | c | d | |||||
| 3 | e | g | f | |||||
��2��e��Ԫ�ط�����Na��
��3��bԪ��ԭ�ӵ�����������Ϊ4��
��4��b��c��d����Ԫ�صķǽ���������ǿ�������������ǿ������
��5��dԪ�غ�fԪ�ؾ����γ���̬�⻯����ȶ���ΪH2d����H2f ������ڡ���С�ڡ�����
��6����������Ԫ�ص�����������ˮ�����У�������ǿ����NaOH��������ǿ����HClO4�������������м��Ե���Al��OH��3��д��ѧʽ��
| A�� | HX��CH3COOH��������ˮ�ĵ��� | |
| B�� | ��HX��CH3COOH ��0.1mol����ˮ���1L�����Һ������Һ��c��H+��=0.2 mol•L-1 | |
| C�� | �������ʵ���Ũ�ȡ��������HX��CH3COONa����Һ��Ϻ�������Һ�У�c��Na+����c��CH3COOH����c��CH3COO-����c��H+�� | |
| D�� | 0.3mol•L-1��CH3COOK��Һ��c��CH3COO-��+c��CH3COOH��=c��K+�� |
| A�� | ���� | B�� | ��ϩ | C�� | �ױ� | D�� | ������ |
| A�� | 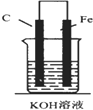 | B�� | 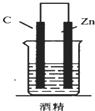 | C�� | 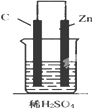 | D�� |  |
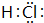 ����������ˮ�����ڹ��ۼ��ܵ��ƻ��������H+��Q-��ʹ��Һ�����ԣ��ṹ������ͬY��W���⻯��ķе�Ӹߵ������д����ǣ��ѧʽ��H2O��H2S��
����������ˮ�����ڹ��ۼ��ܵ��ƻ��������H+��Q-��ʹ��Һ�����ԣ��ṹ������ͬY��W���⻯��ķе�Ӹߵ������д����ǣ��ѧʽ��H2O��H2S��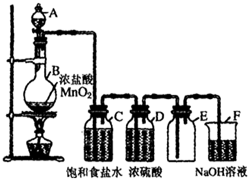 С��ͬѧΪ���Ƶô�����������������Կα��ϵ�ʵ�鷽�������˸Ľ����ڷ���װ�ú��ռ�װ��֮��������ʢ�б���ʳ��ˮ��Ũ�����ϴ��ƿ����ͼ������ش�
С��ͬѧΪ���Ƶô�����������������Կα��ϵ�ʵ�鷽�������˸Ľ����ڷ���װ�ú��ռ�װ��֮��������ʢ�б���ʳ��ˮ��Ũ�����ϴ��ƿ����ͼ������ش�