��Ŀ����
10�������£�0.1mol•L-1HX��pH=1��0.1mol•L-1CH3COOH��pH=2.9������˵������ȷ���ǣ�������| A�� | HX��CH3COOH��������ˮ�ĵ��� | |
| B�� | ��HX��CH3COOH ��0.1mol����ˮ���1L�����Һ������Һ��c��H+��=0.2 mol•L-1 | |
| C�� | �������ʵ���Ũ�ȡ��������HX��CH3COONa����Һ��Ϻ�������Һ�У�c��Na+����c��CH3COOH����c��CH3COO-����c��H+�� | |
| D�� | 0.3mol•L-1��CH3COOK��Һ��c��CH3COO-��+c��CH3COOH��=c��K+�� |
���� A���������ˮ�ĵ��룻
B��������������ڵ���ƽ�������
C���������ʵ���Ũ�ȡ��������HX��CH3COONa����Һ������ɵ����ʵ�����CH3COOH��NaX��c��CH3COO-����c��H+����
D�����������غ�����жϣ�n��K��=n��CH3COO-����
��� �⣺A���������ˮ�ĵ��룬�����£�0.1mol•L-1HX��pH=1˵��HXΪǿ�ᣬ0.1mol•L-1CH3COOH��pH=2.9������Ϊ���ᣬ����ˮ�ĵ������������ã���A��ȷ��
B��������������ڵ���ƽ������������£�0.1mol•L-1HX��pH=1˵��HXΪǿ�ᣬ0.1mol•L-1CH3COOH��pH=2.9������Ϊ���ᣬ��HX��CH3COOH ��0.1mol����ˮ���1L�����Һ������Һ��c��H+����0.2 mol•L-1����B����
C���������ʵ���Ũ�ȡ��������HX��CH3COONa����Һ������ɵ����ʵ�����CH3COOH��NaX����Һ�л�����ˮ�ĵ���ƽ�⣬c��CH3COO-����c��H+������C����
D�����������غ�����жϣ�n��K��=n��CH3COO-����0.3mol•L-1��CH3COOK��Һ��c��CH3COO-��+c��CH3COOH��=c��K+������D��ȷ��
��ѡBC��
���� ���⿼����������ʵ���ƽ���Ӱ�����ط����жϣ�����ˮ��ķ���Ӧ�ã���Ҫ����Һ������Ũ�ȴ�С�Ƚϣ������غ������Ӧ�ã����ջ����ǹؼ�����Ŀ�Ѷ��еȣ�

 Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д� ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�| A�� | C��D��E��A��B | B�� | E��C��D��A��B | C�� | B��A��D��C��E | D�� | B��A��E��D��C |
| A�� | ǿ������Һ�У�NH4+��K+��CO32-��Cl- | |
| B�� | ��SO42-���ڵ���Һ�У�Na+��Mg2+��Ba2+��I- | |
| C�� | ���������ܷų���������Һ�У�K+��Ba2+��Cl-��Br- | |
| D�� | ��ǿ���Ե���Һ�У�NH4+��Na+��Cl-��H+ |
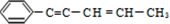 ���й���ṹ˵����ȷ���ǣ�������
���й���ṹ˵����ȷ���ǣ�������| A�� | ����ԭ�ӿ�����ͬһƽ���� | B�� | ����ԭ�Ӳ�������ͬһ��ֱ���� | ||
| C�� | ����̼ԭ�ӿ�����ͬһƽ���� | D�� | �������ڱ���ͬϵ�� |
| A�� | 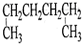 1��4-�������� | B�� | 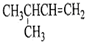 3-����ϩ | C�� | 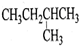 2-������ | D�� | 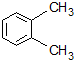 �ڶ��ױ� |
| A�� | һ�ȼ����2-�ȱ��飺������ | |
| B�� | 2��2-����-1-�ȱ�����һ�ȼ��飺2��2-�������� | |
| C�� | 1��5-�������飺������ | |
| D�� | һ�ȼ�������Ȼ�̼��2��2-�������� |
 ��
�� �����������У��뾶��С����Na+�������ӷ��ţ���
�����������У��뾶��С����Na+�������ӷ��ţ��� B2C2
B2C2 ��
��
 +H2O��n HOCH2CH2OH+n HOOC-COOH
+H2O��n HOCH2CH2OH+n HOOC-COOH 
 +��2n-1��H2O��
+��2n-1��H2O��