��Ŀ����
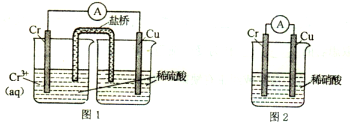
18������ѧ�ḻ��ʣ������Ǹ����ʻ��Ǻ���������������Ϳ�ѧ�о��ж�������Ҫ�ļ�ֵ������������Ϣ�ش��������⣮��1������ͼװ���У��۲쵽ͼ1װ��ͭ�缫�ϲ�����������ɫ���ݣ���ͼ2װ����ͭ�缫����������������缫��������������ɫ���壮���������������Ʋ��������������Ҫ��ѧ���ʽ������Ļ�Ա�ͭǿ���ܺ�ϡ���ᷴӦ���� H2���������ױ�ϡ����ۻ���
��2��Cr��OH��3��Al��OH��3���ƣ�Ҳ���������������ˮ�д�����ʽ�ͼ�ʽ����ƽ�⣬����ʽ���뷽��ʽ��Cr��OH��3?H++CrO2-+H2O��
��3���ƺ�ݳ���������ͨ�¹ʵ���Ҫԭ�����Լ�ʻԱ���к����ƾ�����ԭ���ǣ���ɫ��K2Cr2O7����ˮ��Һ���Ҵ�Ѹ����������ɫCr3+����Ӧԭ��Ϊ��
K2Cr2O7+C2H5OH+H2SO4һK2SO4+Cr2��SO4��3+CH3COOH+H2O
��д������ƽ������ӷ���ʽ2Cr2O72-+3C2H5OH+16H+=4Cr3++3CH3COOH+11H2O��
����Ӧ����0.03mol����ת��ʱ�����������Ҵ���0.0075mol��
��4����ҵ�Ͼ�����������Ⱦ����֮һ�ǣ�����K2Cr2O7���Է�ˮ��������ڣ�����������NaCl����Fe��ʯīΪ�缫���е�⣮����һ��ʱ�������Cr��OH��3��Fe��OH��3������ȥ����֪Ksp[Fe��OH��3]=4.0��10-38��Ksp[Cr��OH��3]=6.0��10-31����
����װ����Ӧ����������������ƣ������ص������������ϵ缫��ӦΪ2H++2e-=H2����
����֪�������Һ��c��Fe3+��Ϊ2.0��10-13mol/L������Һ��c��Cr3+��Ϊ3��10-6mol/L��
���� ��1��ͼ1װ��ͭ�缫�ϲ�����������ɫ���ݣ�˵��Cr�Ļ����Դ���Cu���ܺ�ϡ���ᷴӦ���� H2����ͼ 2װ����ͭ�缫����������������缫�ϲ���������ɫ���壬˵��Cr�������ܲ����ۻ�����
��2�����Al��OH��3����ʽ���������д��
��3�����ݵ��ӵ�ʧ�غ㣬��ƽ���ϵ��������ʽΪ��2K2Cr2O7+3CH3CH2OH+8H2SO4=2Cr2��SO4��3+3CH3COOH+2K2SO4+11H2O��Ȼ����д���ӷ���ʽ����Ӧ��CrԪ�صĻ��ϼ۽��ͣ��Ҵ���CԪ�صĻ��ϼ����ߣ����Ԫ�ػ��ϼ۵ı仯��������ת�������Ӷ�ȷ�����������Ҵ������ʵ����Դ������
��4������������������Ӧ�������������ӣ����Ե缫����Ϊ���������ϵ缫��ӦΪ�������ӷ�����ԭ��Ӧ����������
�ڸ��ݵ�����Һ��c��Fe3+������KsP[Fe��OH��3]������Һ��c��OH-�����ٸ���KsP[Cr��OH��3]������Һ��c��Cr3+����
��� �⣺��1��ͼ1װ��ͭ�缫�ϲ�����������ɫ���ݣ�˵��Cr�Ļ����Դ���Cu���ܺ�ϡ���ᷴӦ���� H2����ͼ 2װ����ͭ�缫����������������缫�ϲ���������ɫ���壬˵��Cr�������ܲ����ۻ�����
�ʴ�Ϊ���������Ļ�Ա�ͭǿ���ܺ�ϡ���ᷴӦ���� H2���������ױ�ϡ����ۻ���
��2��Cr��OH��3��Al��OH��3���ƣ���Al��OH��3����ʽ�����֪��Cr��OH��3����ʽ���뷽��ʽ�ǣ�Cr��OH��3?H++CrO2-+H2O��
�ʴ�Ϊ��Cr��OH��3?H++CrO2-+H2O��
��3�����ݵ��ӵ�ʧ�غ㣬��ƽ���ϵ��������ʽΪ��2K2Cr2O7+3CH3CH2OH+8H2SO4=2Cr2��SO4��3+3CH3COOH+2K2SO4+11H2O���������ӷ���Ϊ��2Cr2O72-+3C2H5OH+16H+=4Cr3++3CH3COOH+11H2O����Ӧ��CrԪ�صĻ��ϼ۽��ͣ�K2Cr2O7��������������������ԭ�õ���ԭ�����Cr2��SO4��3Ϊ��ԭ����Ҵ���CԪ�صĻ��ϼ����ߣ��Ҵ��ǻ�ԭ������ԭ�����л�ԭ�ԣ�K2Cr2O7��CrԪ�ش�+6�۽��͵�+3�ۣ���1mol������ת��6mol���ӣ���������CH3CH2OHΪ1.5mol������0.03mol����ת��ʱ�����������Ҵ�����Ϊ0.0075mol��
�ʴ�Ϊ��2Cr2O72-+3C2H5OH+16H+=4Cr3++3CH3COOH+11H2O��0.0075��
��4������������������Ӧ�������������ӣ����Ե缫����Ϊ���������ϵ缫��ӦΪ�������ӷ�����ԭ��Ӧ�������������������ĵ缫��ӦʽΪ��2H++2e-=H2����
�ʴ�Ϊ������2H++2e-=H2����
�ڵ�����Һ��c��Fe3+��=2.0��10-13mol/L������Һ��c3��OH-��=$\frac{4��1{0}^{-38}}{2��1{0}^{-13}}$mol/L=2��10-25mol/L������Һ��c��Cr3+��=$\frac{6��1{0}^{-31}}{2��1{0}^{-25}}$mol/L=3��10-6mol/L��
�ʴ�Ϊ��3��10-6��
���� ���⿼����������ԭ��Ӧ��Ksp���йؼ��㣬�Լ�ͼ���������Ŀ�Ѷ��еȣ�ע���ͼ��ķ��������ݵĴ�����

 ����ѵ�����⿼ϵ�д�
����ѵ�����⿼ϵ�д� �������ϵ�д�
�������ϵ�д�| A�� | Fe2+ | B�� | Mg2+ | C�� | Al3+ | D�� | Fe3+ |
| A�� | 0.56 L�����к��еĹ��ۼ���Ϊ0.1NA | |
| B�� | ��״���£�1mol SO3���е���ԭ����Ϊ3NA | |
| C�� | 3mol NO2������H2O��Ӧ��ת��1 NA������ | |
| D�� | pH=1��ϡ�����к��е�H+��Ϊ0.1NA |
| A�� | H2��O2 | B�� | SO2��H2S | C�� | NO��O2 | D�� | HCl��NH3 |
| A�� | pH=1����Һ�У�Na+��Fe2+��NO3-��SO42- | |
| B�� | ��Fe3+����Һ�У�K+��Mg2+��S2-��NO3- | |
| C�� | pH=13����Һ��Na+��K+��SiO32-��NO3- | |
| D�� | ǿ������Һ�У�K+��Al3+��Cl-��SO42- |
| A�� | ����Ԫ�ص�ԭ�Ӱ뾶��A��B��D��C | |
| B�� | DԪ�ش���Ԫ�����ڱ��е�3���ڵڢ�A�� | |
| C�� | B��D������������У�B��D����ԭ��֮���Ϊ˫�� | |
| D�� | һ�������£�D�������û���B���ʣ�C�������û���A���� |
| A�� | ���� | B�� | ����� | C�� | �ռ� | D�� | ����ʳ��ˮ |