��Ŀ����
3�� �Ҷ����׳Ʋ��ᣨ�ṹ��ʽΪHOOC-COOH���ɼ�дΪH2C2O4��������һ����Ҫ�Ļ���ԭ�ϣ���������0.01mol/L��H2C2O4��KHC2O4��K2C2O4��Һ��pH�����ʾ����
�Ҷ����׳Ʋ��ᣨ�ṹ��ʽΪHOOC-COOH���ɼ�дΪH2C2O4��������һ����Ҫ�Ļ���ԭ�ϣ���������0.01mol/L��H2C2O4��KHC2O4��K2C2O4��Һ��pH�����ʾ����| H2C2O4 | KHC2O4 | K2C2O4 | |
| pH | 2.1 | 3.1 | 8.1 |
��1��д��H2C2O4�ĵ��뷽��ʽH2C2O4?H++HC2O4-��HC2O4-?H++C2O42-����
��2��KHC2O4��Һ�����Ե�ԭ����HC2O4-�ĵ���̶ȴ���ˮ��̶ȣ���0.1mol/L�IJ��������Һ��μ�NaOH��Һ�����ԣ���ʱ��Һ�������Ũ�ȹ�ϵ��ȷ����ad��
a��c��K+��=c��HC2O4-��+c��H2C2O4��+c��C2O42-��
b��c��Na+��=c��H2C2O4��+c��C2O42-��
c��c��K+��+c��Na+��=c��HC2O4-��+c��C2O42-��
d��c��K+����c��Na+��
��3��H2C2O4�����Ը��������Һ��Ӧ�������������ݣ�CO2����������ɫ��ʧ��д����Ӧ�����ӷ���ʽ2MnO4-+5H2C2O4+6H+��2Mn2++10CO2��+8H2O����֪�÷�Ӧ��ʼʱ���ʽ����������ӿ죬���ܵ�ԭ���Ƿ�Ӧ���ɵ�Mn2+�Ը÷�Ӧ���д����ã�
��4��ijͬѧ���ʵ����ͼ��ʾ�������ձ��е��Թܶ��ֱ�ʢ��2mL 0.1 mol/L H2C2O4��Һ��4mL 0.1mol/L ����KMnO4��Һ���ֱ��ϲ�����¼��Һ��ɫ����ʱ�䣮��ʵ��Ŀ�����о��¶ȶԷ�Ӧ���ʵ�Ӱ�죬����ʵ��ʼ��û�п�����Һ��ɫ���Ʋ�ԭ��KMnO4��Һ������
��5����֪���ᣨH2C2O4�����ȷֽ�Ļ�ѧ����ʽΪ��H2C2O4��H2O+CO��+CO2����д��FeC2O4���ܱ������и��·ֽ�Ļ�ѧ����ʽFeC2O4$\frac{\underline{\;����\;}}{\;}$Fe+2CO2����
���� ��1��������0.01mol/L��H2C2O4pHΪ2.1��KHC2O4����pHΪ3.1��˵�������Ƕ�Ԫ���
��2��HC2O4-���ܹ�����Ҳ�ܹ�ˮ�⣬KHC2O4��Һ�����ԣ�˵��HC2O4-�ĵ���̶ȴ���ˮ��̶ȣ�
a�����������غ��жϣ�
b�����ݵ���غ��жϣ�
c�����������غ㡢����غ��жϣ�
d����ǡ�÷�Ӧ��c��K+��=c��Na+������ʱ��Һ�ʼ��ԣ��ʼ�����������Ƶ����ʵ�������Щ��
��3��������������Һ��Ӧ��������Һ�� ����������ԭ��Ӧ�����ᱻ����Ϊ������̼��������ر���ԭΪ�����ӣ���Ӧ��ʼʱ���ʽ����������ӿ죬˵�����ɵ�Mn2+�Ը÷�Ӧ���д����ã�
��4�����ձ���ˮ���¶Ȳ�ͬ����ʵ�����о��¶ȶԷ�Ӧ���ʵ�Ӱ�죻�����������ط�Ӧ�����ʵ���֮��Ϊ5��2���ݴ˷�����
��5����֪���ᣨH2C2O4�����ȷֽ�Ļ�ѧ����ʽΪ��H2C2O4��H2O+CO��+CO2��������ֽ��ܹ�����CO��CO���л�ԭ�ԣ��ܹ���ԭFeO��
��� �⣺��1����Ԫ����ֲ����룬������뷽��ʽΪ��H2C2O4?H++HC2O4-��HC2O4-?H++C2O42-���ʴ�Ϊ��H2C2O4?H++HC2O4-��HC2O4-?H++C2O42-��
��2��HC2O4-���ܹ�����Ҳ�ܹ�ˮ�⣬KHC2O4��Һ�����ԣ�˵��HC2O4-�ĵ���̶ȴ���ˮ��̶ȣ�
a��̼Ԫ������Һ�д�����ʽ�У�HC2O4-��H2C2O4��C2O42-�����������غ���c��K+��=c��HC2O4-��+c��H2C2O4��+c��C2O42-������a��ȷ��
b�����ݵ���غ��У�c��K+��+c��Na+��+c��H+��=c��HC2O4-��+2c��C2O42-��+c��OH-������Һ�����ԣ���c��H+��=c��OH-������c��K+��+c��Na+��=c��HC2O4-��+2c��C2O42-������b����
c����c��K+��=c��HC2O4-��+c��H2C2O4��+c��C2O42-����c��K+��+c��Na+��=c��HC2O4-��+2c��C2O42-����֪��c��Na+��=c��C2O42-��-c��H2C2O4������c����
d����ǡ�÷�Ӧ��c��K+��=c��Na+������ʱ��Һ�ʼ��ԣ��ʼ�����������Ƶ����ʵ�������Щ����c��K+����c��Na+������d��ȷ��
�ʴ�Ϊ��HC2O4-�ĵ���̶ȴ���ˮ��̶ȣ�ad��
��3��������������Һ��Ӧ��������Һ�� ����������ԭ��Ӧ�����ᱻ����Ϊ������̼��������ر���ԭΪ�����ӣ���Ӧ�����ӷ���ʽΪ��5H2C2O4+2MnO4-+6H+=2Mn2++10CO2��+8H2O����Ӧ��ʼʱ���ʽ����������ӿ죬˵�����ɵ�Mn2+�Ը÷�Ӧ���д����ã�
�ʴ�Ϊ��2MnO4-+5H2C2O4+6H+��2Mn2++10CO2��+8H2O����Ӧ���ɵ�Mn2+�Ը÷�Ӧ���д����ã�
��4�����ձ���ˮ���¶Ȳ�ͬ����ʵ�����о��¶ȶԷ�Ӧ���ʵ�Ӱ�죻�����������ط�Ӧ�����ʵ���֮��Ϊ5��2���Թ��в��������������ʵ���֮��Ϊ1��2��������ع�����������ȫ��Ӧ�����Բ���ɫ��
�ʴ�Ϊ���¶ȶԷ�Ӧ���ʵ�Ӱ�죻KMnO4��Һ������
��5����֪���ᣨH2C2O4�����ȷֽ�Ļ�ѧ����ʽΪ��H2C2O4��H2O+CO��+CO2����������������ȷֽ�Ļ�ѧ����ʽΪFeC2O4$\frac{\underline{\;����\;}}{\;}$FeO+CO��+CO2����CO�ܹ���ԭFeO��FeO+CO $\frac{\underline{\;����\;}}{\;}$ Fe+CO2���������䷴Ӧ����ʽΪ��FeC2O4$\frac{\underline{\;����\;}}{\;}$ Fe+2CO2����
�ʴ�Ϊ��FeC2O4$\frac{\underline{\;����\;}}{\;}$ Fe+2CO2����
���� ����ͨ�����ῼ���˶�Ԫ����ĵ��뷽��ʽ��д���������Һ�еĵ���غ�������غ㡢����Ļ�ԭ���Լ�Ӱ�컯ѧ��Ӧ���ʵ����ص�̽������Ŀ�ѶȽϴ�

| A�� | ��ϵͳ���������л��� 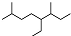 ������Ϊ3��7-����-4-�һ����� ������Ϊ3��7-����-4-�һ����� | |
| B�� | �����ʵ����ı��ͱ�������ȫȼ������������������� | |
| C�� | ����ױ���Ϊͬϵ�����ʹKMnO4������Һ��ɫ | |
| D�� | �ṹƬ��Ϊ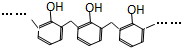 �ĸ߾���䵥���Ǽ�ȩ�ͱ��� �ĸ߾���䵥���Ǽ�ȩ�ͱ��� |
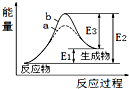
| A�� | �÷�ӦΪ���ȷ�Ӧ | |
| B�� | b��ʾ���д��� | |
| C�� | �����ܽ��������淴Ӧ�Ļ�� | |
| D�� | �淴Ӧ�Ļ�ܴ�������Ӧ�Ļ�� |
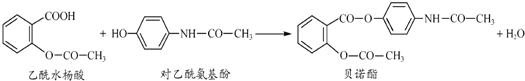
�����й�������ȷ���ǣ�������
| A�� | 1mol��ŵ�����ӿ���6molH2��ȫ��Ӧ | |
| B�� | ����FeCl3 ��Һ��������ˮ����Ͷ����������� | |
| C�� | ��ŵ��������NaOH ��Һ���ȣ�������������ˮ�����ƺͶ������������� | |
| D�� | ����ˮ����Ͷ����������Ӿ�����NaHCO3 ��Һ��Ӧ |
| A�� | ���Ȼ�����Һ�м�������İ�ˮ | |
| B�� | ��ϡ�����е���������NaAlO2��Һ | |
| C�� | ��ϡ�����м������� | |
| D�� | �������ữ��MgSO4��Һ�м��������Ba��OH��2��Һ |
| | X | | Y | |
| R |
| A�� | ����Ԫ����ԭ�Ӱ뾶������W�����Ӱ뾶������R���� | |
| B�� | X��R��W����Ԫ�ص��������������Ӧ��ˮ���������ǿ����ϵΪR��W��X | |
| C�� | Y��Z�γɵĻ�����һ���Ǽ��������� | |
| D�� | Z��W�γɵĻ�����ˮ��Һ���������� |
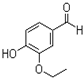 �һ���������һ�ֺϳ����ϣ���ṹ��ʽ��ͼ��
�һ���������һ�ֺϳ����ϣ���ṹ��ʽ��ͼ��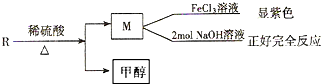
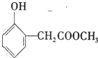 ��
��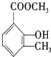 ��
��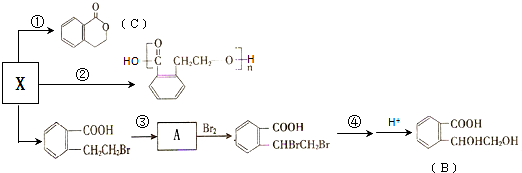
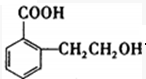 ��B�к��������ŵ������ǻ����Ȼ���
��B�к��������ŵ������ǻ����Ȼ��� ��
�� �����ں��е���������ACE������ţ���
�����ں��е���������ACE������ţ���