��Ŀ����
15��������������ǵĻ������ڿ�ѧ�о���ҵ�����о���������;���ش������й�������1�������йص�˵����ȷ����B
A����һ�����ܴ�С��S��P��Si
B����Ϊ������CaO��KCl�ߣ�����KCl��CaO�۵��
C��SO2��CO2�Ļ�ѧ�������ƣ����ӽṹҲ����ֱ���ͣ���ͬ������SO2���ܽ�ȸ���
D�����Ӿ����У����ۼ�����Խ�÷��Ӿ�����۷е�Խ��
��2������Ni�����γɶ��������Ҹ���������й㷺����;��ij�������ṹ��ͼ��ʾ��
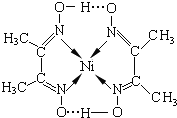 �����ں��е���������ACE������ţ���
�����ں��е���������ACE������ţ���A���������������B�����Ӽ���������C�����ۼ�
D��������������E�����
��ɸ�����������ͬ���ڶ�����Ԫ�صĵ縺���ɴ�С��˳����O��N��C������-CH3����Cԭ�ӵ��ӻ���ʽΪsp3��
��3������ͭ������������������ҪӦ�ã���̬Fe2+��M������Ų�ʽΪ3s23p63d5���þ����x�������䷢���Բ�ð���٤���������Խ���ͭ�IJⶨ�õ����½��������Ϊ�����������ܶѻ����߳�Ϊ361pm����ʾ��1pm=10-10cm��3.613=47.05������֪ͭ���ܶ�Ϊ9.00g•cm-3����ͭ������������4.23��10-22g��������λС�����������ӵ�����Ϊ6.05��1023mol-1��������λС������
���� ��1��A��ͬһ����Ԫ���У�Ԫ�صĵ�һ����������ԭ����������������������ƣ�����VA��Ԫ�صĵ�һ�����ܴ�������Ԫ�أ�
B�������������Ӿ�����۵�����ȣ����Ӱ뾶�뾧���ܳɷ��ȣ�������������뾧���ܳ����ȣ�
C�����������������ԭ�ӵļ۲���Ӷ���3�Һ���һ�����Ӷԣ����Զ���������V�νṹ��Ϊ���Է��ӣ�������������ԭ���жϣ�
D�����Ӿ����У����ʵ��۷е�������Է���������ض��빲�ۼ������أ�
��2�����������Ľṹͼ���жϼ������ͣ�ͬ���ڷǽ���Խǿ�縺��Խǿ�����е�̼ԭ�ӵļ۲���Ӷ����ж��ӻ���ʽ��
��3��Feԭ�Ӻ�����26�����ӣ�Fe3+�����Ų�ʽΪ1s22s22p63s23p63d5���ݴ�д��M������Ų�ʽ�����ݾ����ı߳�����þ��������������������=������ܶȣ�����ͭ����Ϊ�����������ܶѻ���ÿ����������4��ͭԭ�ӣ�ͭ��Ħ������=1/4������������NA���ݴ����NA��
��� �⣺��1��A��ͬһ����Ԫ���У�Ԫ�صĵ�һ����������ԭ����������������������ƣ�����VA��Ԫ�صĵ�һ�����ܴ�������Ԫ�أ����Ե�һ�����ܴ�С��P��S��Si����A����
B�������������Ӿ�����۵�����ȣ����Ӱ뾶�뾧���ܳɷ��ȣ�������������뾧���ܳ����ȣ������ӵ�ɴ��ڼ����ӣ��Ҹ����Ӱ뾶С�ڼ����ӣ��������������С�������ӣ��������Ӱ뾶���������ӣ������Ȼ��صľ�����С�������ƣ����Ȼ��ص��۵�������Ƶͣ���B��ȷ��
C�����������������ԭ�ӵļ۲���Ӷ���3�Һ���һ�����Ӷԣ����Զ���������V�νṹ��Ϊ���Է��ӣ�������̼�ǷǼ��Է��ӣ�������������ԭ��֪����������������ˮ����C����
D�����Ӿ����У����ʵ��۷е�������Է���������ض��빲�ۼ������أ���D����
��ѡB��
��2�����ĵ縺�Ժ�ǿ��������һ���ǻ��γ������������к�̼������̼�����̼��˫���ȹ��ۼ�������������λ�����������Ӽ�Ҳ���ǽ���������ѡACE��ͬ���ڷǽ���Խǿ�縺��Խǿ�����Ե縺��O��N��C�����е�̼ԭ�ӵļ۲���Ӷ���Ϊ$\frac{4+3+1}{2}$=4�����Լ��е�̼ԭ����sp3�ӻ���
�ʴ�Ϊ��ACE��O��N��C��sp3��
��3��Feԭ�Ӻ�����26�����ӣ���������Ų�Ϊ1s22s22p63s23p63d64s2��Feԭ��ʧȥ4s�ܼ�2�����ӡ�3d�ܼ�1�������γ�Fe3+��Fe3+�����Ų�ʽΪ1s22s22p63s23p63d5����M������Ų�ʽΪ��3s23p63d5��1pm=10-10cm����һ�����������Ϊ��361��10-10cm��3=4.7��10-23cm3������������=������ܶȣ���һ������������Ϊ4.7��10-23cm3��9.00g•cm-3=4.23��10-22g������ͭ����Ϊ�����������ܶѻ���ÿ����������4��ͭԭ�ӣ�ͭ��Ħ������=1/4������������NA������64g•mol-1=1/4��4.23��10-22g��NA����NA=6.05��1023mol-1��
�ʴ�Ϊ��3s23p63d5��4.23��10-22��6.05��1023mol-1��
���� ���⿼����ۺϣ��漰�����Ų�ʽ���縺�Դ�С�Ƚϡ���һ�����ܡ��ӻ�����������ļ����֪ʶ�㣬�����ܶȹ�ʽ��Ԫ�������ɵ�֪ʶ������������ѵ��Ǿ����ļ��㣬��Ŀ�Ѷ��еȣ�

 ��������ϵ�д�
��������ϵ�д� ���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д� �Ҷ����׳Ʋ��ᣨ�ṹ��ʽΪHOOC-COOH���ɼ�дΪH2C2O4��������һ����Ҫ�Ļ���ԭ�ϣ���������0.01mol/L��H2C2O4��KHC2O4��K2C2O4��Һ��pH�����ʾ����
�Ҷ����׳Ʋ��ᣨ�ṹ��ʽΪHOOC-COOH���ɼ�дΪH2C2O4��������һ����Ҫ�Ļ���ԭ�ϣ���������0.01mol/L��H2C2O4��KHC2O4��K2C2O4��Һ��pH�����ʾ����| H2C2O4 | KHC2O4 | K2C2O4 | |
| pH | 2.1 | 3.1 | 8.1 |
��1��д��H2C2O4�ĵ��뷽��ʽH2C2O4?H++HC2O4-��HC2O4-?H++C2O42-����
��2��KHC2O4��Һ�����Ե�ԭ����HC2O4-�ĵ���̶ȴ���ˮ��̶ȣ���0.1mol/L�IJ��������Һ��μ�NaOH��Һ�����ԣ���ʱ��Һ�������Ũ�ȹ�ϵ��ȷ����ad��
a��c��K+��=c��HC2O4-��+c��H2C2O4��+c��C2O42-��
b��c��Na+��=c��H2C2O4��+c��C2O42-��
c��c��K+��+c��Na+��=c��HC2O4-��+c��C2O42-��
d��c��K+����c��Na+��
��3��H2C2O4�����Ը��������Һ��Ӧ�������������ݣ�CO2����������ɫ��ʧ��д����Ӧ�����ӷ���ʽ2MnO4-+5H2C2O4+6H+��2Mn2++10CO2��+8H2O����֪�÷�Ӧ��ʼʱ���ʽ����������ӿ죬���ܵ�ԭ���Ƿ�Ӧ���ɵ�Mn2+�Ը÷�Ӧ���д����ã�
��4��ijͬѧ���ʵ����ͼ��ʾ�������ձ��е��Թܶ��ֱ�ʢ��2mL 0.1 mol/L H2C2O4��Һ��4mL 0.1mol/L ����KMnO4��Һ���ֱ��ϲ�����¼��Һ��ɫ����ʱ�䣮��ʵ��Ŀ�����о��¶ȶԷ�Ӧ���ʵ�Ӱ�죬����ʵ��ʼ��û�п�����Һ��ɫ���Ʋ�ԭ��KMnO4��Һ������
��5����֪���ᣨH2C2O4�����ȷֽ�Ļ�ѧ����ʽΪ��H2C2O4��H2O+CO��+CO2����д��FeC2O4���ܱ������и��·ֽ�Ļ�ѧ����ʽFeC2O4$\frac{\underline{\;����\;}}{\;}$Fe+2CO2����
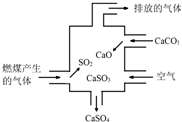
| A�� | ʹ�ô�װ�ÿ��Լ��ٵ��������������ŷ� | |
| B�� | ��װ���ڼȷ����˻��Ϸ�Ӧ��Ҳ�����˷ֽⷴӦ | |
| C�� | �ܷ�Ӧ�ɱ�ʾΪ��2SO2+2CaCO3+O2��2CaSO4+2CO2 | |
| D�� | ���ŷŵ�������ʹ����ʯ��ˮ����ǣ�˵���������к�SO2 |
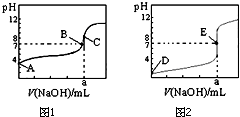 ��ͼ1��2Ϊ��������0.10mol•L-1 NaOH��Һ�ζ�20.00mL 0.10mol•L-1 �����20.00mL 0.10mol•L-1��������ߣ�����HA��ʾ�ᣬ�����жϺ�˵����ȷ���ǣ�������
��ͼ1��2Ϊ��������0.10mol•L-1 NaOH��Һ�ζ�20.00mL 0.10mol•L-1 �����20.00mL 0.10mol•L-1��������ߣ�����HA��ʾ�ᣬ�����жϺ�˵����ȷ���ǣ�������| A�� | ͼ1�ǵζ���������� | |
| B�� | E��ʱ��Һ������Ũ��Ϊc��Na+ ��=c��A- �� | |
| C�� | B��ʱ����Ӧ������Һ�����V��NaOH����V��HA�� | |
| D�� | ��0mL��V��NaOH����20.00mLʱ����Һ��һ����c��A-����c��Na+ ����c��H+ ����c��OH- �� |
| X | Y | ||
| Z | W |
| A�� | ԭ�Ӱ뾶��С�����˳��ΪX��Z��Y��W | |
| B�� | YԪ������������Ӧ��ˮ���ﻯѧʽΪHYO3 | |
| C�� | X��Z����Ԫ�ص���������������ѧ��������ͬ | |
| D�� | Y�����̬�⻯���Y��������ͬ����Ԫ����̬�⻯��е�� |
| A�� | ���������� | B�� | ��Һ��Ca2+����Ŀ���� | ||
| C�� | �ܼ���������С | D�� | ��Һ��pH���� |
 ���磺
���磺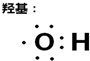
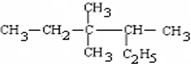 ������3��3��4-��������
������3��3��4-��������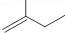 ������2-��-1-��ϩ��
������2-��-1-��ϩ��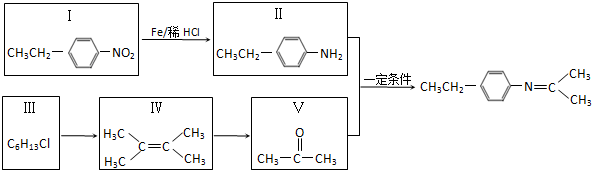
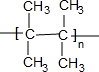 ��
��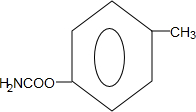 ��д��һ�֣���
��д��һ�֣���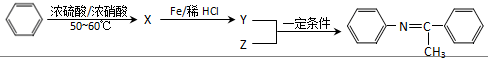
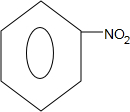 ��Z�Ľṹ��ʽΪ
��Z�Ľṹ��ʽΪ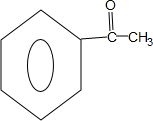 ��
��