��Ŀ����
18��ijѧϰС��Ϊ�ⶨ�����Ѿõ�С�մ���Ʒ�д���������������������ʵ�飺��1������һ����ȡһ����������Ʒ�����������м��������غ���ȴ����ȡʣ���������������
����ɱ�ʵ����Ҫ�����ò��������裬��Ŀ����ʹ�������Ⱦ��ȣ�����ֲ��¶ȹ��ߣ���ɹ����⽦
��ʵ���м��������ص�Ŀ����ʹNaHCO3�ֽ���ȫ����ʹˮ��ȫ�ӷ���
����ʵ��ǰ������Ʒ������Ϊmg������������ʱ��������Ϊag������Ʒ�д������������Ϊ$\frac{84a-53m}{31m}$��100%��
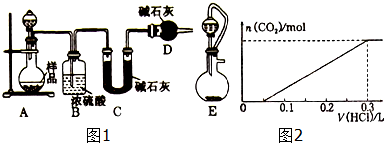
��2��������������ͼ1��ʾװ�ý���ʵ�飬���ش��������⣺
��ʵ��ǰ�ȼ��װ�õ������ԣ�����ȡһ����������Ʒ����A�У���ϡ����װ���Һ©���У�Dװ�õ����������տ����еĶ�����̼��ˮ��������������еĶ�����̼��ˮ��������C�У�
��ʵ���г�������Ʒ�����⣬����ֱ����Cװ�÷�Ӧǰ�����������
����ͬѧ��Ϊ����Eװ�����Aװ�������ʵ��ȷ�ȣ�����Ϊ�Ƿ���ȷ�������Ƿ�Eװ���ú�ѹ��Һ©�������ֶ�����̼Ϊ�����ڷ�Һ©���ϲ���ʹC�����ն�����̼������С����ɽϴ�����
��3������������ȡһ��������Ʒ������ƿ�У�������ˮ����������еζ����ӿ�ʼ����������������岻�ٲ������μӵ����������ͼ2��ʾ����С�մ���Ʒ�д������������Ϊ23.98%����
���� С�մ���ûᷢ����Ӧ��2NaHCO3$\frac{\underline{\;\;��\;\;}}{\;}$Na2CO3+CO2��+H2O������Ʒ�ɷ�ΪNaHCO3��Na2CO3���ⶨ��Ʒ�д�����������������У��ⶨ������̼���������ⶨ̼���Ƶ��������ⶨ̼�����Ƶ�������
��1����ʹ�ò��������裬����ֲ��¶ȹ��߶��ǹ���ɽ���
�ڼ��������ص�Ŀ����ʹNaHCO3�ֽ���ȫ����ʹˮ��ȫ�ӷ���
�ۼ���̼�����Ʒֽ�����̼���ƣ�����Ʒ��NaHCO3��Na2CO3�Ļ����ת��ΪNa2CO3�����ݹ�����������÷���ʽ���ò�����������Ʒ��̼�����Ƶ���������������̼���Ƽ���̼���Ƶ�����������
��2��������C�м�ʯ�����زⶨ��Ӧ���ɶ�����̼������������������Ʒ��̼���Ƶ��������������ڼ�ʯ�ҿ������տ����еĶ�����̼��ˮ��������Dװ�õ������DZ�������еĶ�����̼��ˮ��������C�У�
��Cװ�÷�Ӧǰ������֮��Ϊ��Ӧ���ɶ�����̼��������������Ʒ��������������̼���������Լ���������̼���Ƶ�������
��Eװ���ú�ѹ��Һ©�������ֶ�����̼Ϊ�����ڷ�Һ©���ϲ���ʹC�����ն�����̼������С��
��4����ͼ��֪����ʼ������Ӧ��Na2CO3+HCl=NaHCO3������������̼�ķ�ӦΪ��HCl+NaHCO3$\frac{\underline{\;\;��\;\;}}{\;}$NaCl+CO2��+H2O��������ÿ���̶�Ϊ50mL����ÿ���̶�Ϊ1molHCl�����ݷ���ʽ����̼���Ƶ����ʵ������ټ�����Ʒ��̼�����Ƶ����ʵ�������������̼���Ƶ�����������
��� �⣺С�մ���ûᷢ����Ӧ��2NaHCO3$\frac{\underline{\;\;��\;\;}}{\;}$Na2CO3+CO2��+H2O������Ʒ�ɷ�ΪNaHCO3��Na2CO3���ⶨ��Ʒ�д�����������������У��ⶨ������̼���������ⶨ̼���Ƶ��������ⶨ̼�����Ƶ�������
��1����ʹ�ò��������裬ʹ�������Ⱦ��ȣ�����ֲ��¶ȹ��ߣ���ɹ����⽦��
�ʴ�Ϊ��ʹ�������Ⱦ��ȣ�����ֲ��¶ȹ��ߣ���ɹ����⽦��
�ڼ��������ص�Ŀ����ʹNaHCO3�ֽ���ȫ����ʹˮ��ȫ�ӷ�������Ʒ��NaHCO3��Na2CO3�Ļ����ת��ΪNa2CO3��
�ʴ�Ϊ��ʹNaHCO3�ֽ���ȫ����ʹˮ��ȫ�ӷ���
������Ʒ��̼�����Ƶ�����Ϊx����
2NaHCO3$\frac{\underline{\;\;��\;\;}}{\;}$Na2CO3+CO2��+H2O ��������
168 62
x ��m-a��g
��x=$\frac{168��m-a��g}{62}$=$\frac{84��m-a��}{31}$g����m��Na2CO3��=[m-$\frac{84��m-a��}{31}$]g��
����Ʒ��Na2CO3����������Ϊ{[m-$\frac{84��m-a��}{31}$]g��mg}��100%=$\frac{84a-53m}{31m}$��100%��
�ʴ�Ϊ��$\frac{84a-53m}{31m}$��100%��
��2��������C�м�ʯ�����زⶨ��Ӧ���ɶ�����̼������������������Ʒ��̼���Ƶ��������������ڼ�ʯ�ҿ������տ����еĶ�����̼��ˮ��������Dװ�õ����������տ����еĶ�����̼��ˮ��������������еĶ�����̼��ˮ��������C�У���ֹ�ⶨ��
�ʴ�Ϊ�����տ����еĶ�����̼��ˮ��������������еĶ�����̼��ˮ��������C�У�
��Cװ�÷�Ӧǰ������֮��Ϊ��Ӧ���ɶ�����̼��������������Ʒ��������������̼���������Լ���������̼���Ƶ�����������ֱ����Cװ�÷�Ӧǰ�����������
�ʴ�Ϊ��C��
��Eװ���ú�ѹ��Һ©�������ֶ�����̼Ϊ�����ڷ�Һ©���ϲ���ʹC�����ն�����̼������С����ɽϴ����
�ʴ�Ϊ��Eװ���ú�ѹ��Һ©�������ֶ�����̼Ϊ�����ڷ�Һ©���ϲ���ʹC�����ն�����̼������С����ɽϴ����
��4����ͼ��֪����ʼ������Ӧ��Na2CO3+HCl=NaHCO3������������̼�ķ�ӦΪ��HCl+NaHCO3$\frac{\underline{\;\;��\;\;}}{\;}$NaCl+CO2��+H2O��������ÿ���̶�Ϊ50mL����ÿ���̶�Ϊ1molHCl���ɷ���ʽ��֪����Ʒ��n��Na2CO3��=1mol��̼���Ʒ�Ӧ��̼������Ϊ1mol����ԭ��Ʒ��̼�����Ƶ����ʵ���Ϊ5mol-1mol=4mol����ԭ�������̼���Ƶ���������Ϊ$\frac{1mol��106g/mol}{1mol��106g/mol+4mol��84g/mol}$��100%=23.98%��
�ʴ�Ϊ��23.98%��
���� ���⿼��������ɺ����IJⶨ����ȷʵ��ԭ���ǽ���ؼ����Ƕ�ѧ���ۺ������Ŀ��飬�Ѷ��еȣ�ע�ⷽ������һ����ȱ�ݣ�װ���еĶ�����̼δ����ȫ��C�м�ʯ�����գ�

 �����Ļ�������ҵϵ�д�
�����Ļ�������ҵϵ�д�| A�� | �������ʵ�����SO2�����Cl2ͬʱ������ʪ��ĺ�ɫ������Ư��Ч�������� | |
| B�� | SO2�����Cl2 Ư��ԭ����ͬ | |
| C�� | SO2�����Cl2��������� | |
| D�� | SO2ֻ��������û�л�ԭ�� |
 ç������һ�ֺϳ�ҩ���Ƶ�ԭ�ϣ����������ƻ������ʯ���ֲ���У����й����������л��������˵����ȷ���ǣ�������
ç������һ�ֺϳ�ҩ���Ƶ�ԭ�ϣ����������ƻ������ʯ���ֲ���У����й����������л��������˵����ȷ���ǣ�������| A�� | �����������Ȼ�����Һ����ɫ | |
| B�� | ������ӱ�ç������Ӷ�����˫�� | |
| C�� | �����ʵ�������������NaOH��Ӧ������NaOH������ͬ | |
| D�� | �����ᶼ������ˮ��Ӧ |
| A�� | ��֬�Dz���������ߵ�Ӫ������ | |
| B�� | ���ܷ���������Ӧ | |
| C�� | ��ά�ؿ�������������ˮ��������� | |
| D�� | �Ȱ�������к���2�ֹ����� |
| A�� | ��ˮ�Ͷ�������ʹƷ����Һ��ɫ | |
| B�� | ϡ��������Ȼ�����ҺʹKI-������ֽ���� | |
| C�� | �������ƺ�ˮ�������ڱ�¶�ڿ����б��� | |
| D�� | Ũ�����Ũ���᳤�ڱ�¶�ڿ�����Ũ�ȱ�С |
| A�� | ������ڿ����м��Ⱦ������ɶ��������� | |
| B�� | �������ˮ��Ӧ��������ˮ���ϣ� | |
| C�� | ±�ظ����ʶ��ܺ�ˮ���ҷ�Ӧ�� | |
| D�� | ±�ص���Խ���ã����۷е��Խ�� |
| A�� | C3N4�Ƿ��Ӿ��� | |
| B�� | C3N4��������ͨ�����Ӽ���� | |
| C�� | C3N4������е����Ժ���չ�� | |
| D�� | C3N4��������C��Nԭ��Ϊ�����������ۼ�Ϊ�������Ŀռ���״�ṹ |
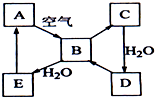 ��ͼ��ʾ����ɫ��̬����A���仯����֮���ת����ϵ��ijЩ����ͷ�Ӧ��������ȥ����������B�ڳ��³�ѹ��Ϊ���壬B��C����Է�������֮��Ϊ4��5��������D����Ҫ�Ĺ�ҵԭ�ϣ�
��ͼ��ʾ����ɫ��̬����A���仯����֮���ת����ϵ��ijЩ����ͷ�Ӧ��������ȥ����������B�ڳ��³�ѹ��Ϊ���壬B��C����Է�������֮��Ϊ4��5��������D����Ҫ�Ĺ�ҵԭ�ϣ�