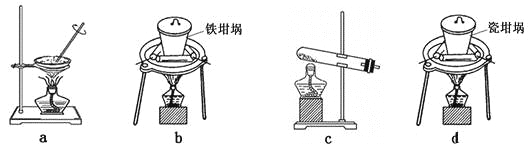
��Ŀ����
����Ŀ����ҵ�����в�����![]() ��NOֱ���ŷŽ��Դ������������Ⱦ�����õ绯ѧԭ������
��NOֱ���ŷŽ��Դ������������Ⱦ�����õ绯ѧԭ������![]() ��NO��ͬʱ���
��NO��ͬʱ���![]() ��
��![]() ��Ʒ�Ĺ�������ͼ����
��Ʒ�Ĺ�������ͼ����![]() Ϊ��Ԫ��
Ϊ��Ԫ��![]() ��
��
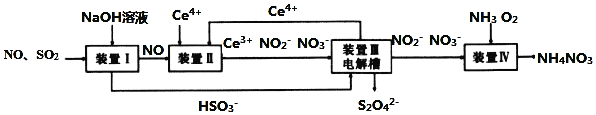
��ش��������⣮
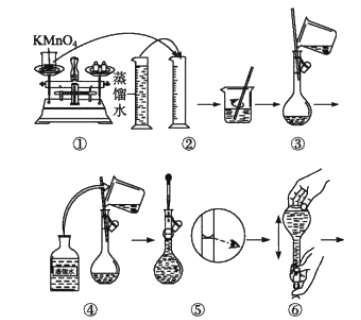
![]() ��װ�â��з�Ӧ���ɵĺ�������������ʵ�����Ϊ1��1����÷�Ӧ�����ӷ���ʽ____��
��װ�â��з�Ӧ���ɵĺ�������������ʵ�����Ϊ1��1����÷�Ӧ�����ӷ���ʽ____��
![]() �������
�������![]() ��
��![]() ��
��![]() ������
������![]() ��NaOH��Һ��Ӧ�����Һ�У����ǵ����ʵ�������
��NaOH��Һ��Ӧ�����Һ�У����ǵ����ʵ�������![]() ����ҺpH�Ĺ�ϵ��ͼ��ʾ��
����ҺpH�Ĺ�ϵ��ͼ��ʾ��
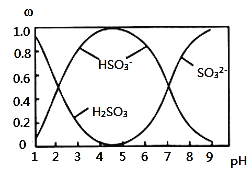
![]() ����˵������ȷ����______
����˵������ȷ����______![]() ����
����![]() ��
��
A.![]() ��Һ�еμ���ˮ����ʹ
��Һ�еμ���ˮ����ʹ![]() ��ֵ���
��ֵ���
B.![]() ʱ����Һ��
ʱ����Һ��![]()
C.![]() ʱ����Һ��
ʱ����Һ��![]()
D.��ͼ�����ݣ����Թ����![]() �ĵڶ�������ƽ�ⳣ��
�ĵڶ�������ƽ�ⳣ��![]()
E.��ͼ�����ݣ�![]() ��
��![]() ʱ����Һ��ˮ�ĵ���̶���ͬ
ʱ����Һ��ˮ�ĵ���̶���ͬ
![]() �����ð�ˮ���ն�����������ط�Ӧ����Ҫ�Ȼ�ѧ����ʽ���£�
�����ð�ˮ���ն�����������ط�Ӧ����Ҫ�Ȼ�ѧ����ʽ���£�
![]()
![]()
![]()
![]()
![]()
![]()
![]()
��Ӧ![]() ___
___![]() ��
��
![]() ȡװ�â��е�����ҺVmL����
ȡװ�â��е�����ҺVmL����![]() �����Ը��������Һ�ζ�������Һ��ҺӦװ��______
�����Ը��������Һ�ζ�������Һ��ҺӦװ��______![]() ������ʽ��������ʽ��
������ʽ��������ʽ��![]() �ζ����У��жϵζ��յ�ķ���______��
�ζ����У��жϵζ��յ�ķ���______��
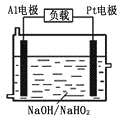
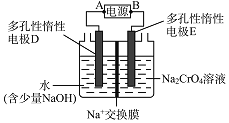
![]() װ�â������֮һ������
װ�â������֮һ������![]() ����ԭ����ͼ��ʾ��ͼ��BΪ��Դ��______
����ԭ����ͼ��ʾ��ͼ��BΪ��Դ��______![]() ����������������
����������������![]() ������෴Ӧ���з�������Ҫ�缫��ӦʽΪ______��
������෴Ӧ���з�������Ҫ�缫��ӦʽΪ______��
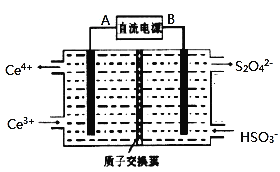
![]() ��֪����װ�â�����Һ��
��֪����װ�â�����Һ��![]() ��Ũ��Ϊ
��Ũ��Ϊ![]() ��Ҫʹ
��Ҫʹ![]() ����Һ�е�
����Һ�е�![]() ��ȫת��Ϊ
��ȫת��Ϊ![]() ���ù�����ת�Ƶ�����ĿΪ______��
���ù�����ת�Ƶ�����ĿΪ______��
���𰸡�![]() BD
BD ![]() ��ʽ ���һ����Һ��Ϊ��ɫ�Ұ���Ӳ���˵����Ӧ�ﵽ�յ� ��
��ʽ ���һ����Һ��Ϊ��ɫ�Ұ���Ӳ���˵����Ӧ�ﵽ�յ� �� ![]()
![]()
��������
װ�â��ж�������������������ܺ�ǿ����������֮�䷢����Ӧ��![]() ��NO����������֮�䲻�ᷴӦ��װ�â���NO�����������£�NO��
��NO����������֮�䲻�ᷴӦ��װ�â���NO�����������£�NO��![]() ֮��ᷢ��������ԭ��Ӧ��
֮��ᷢ��������ԭ��Ӧ��![]() ��
��![]() ��װ�â��У��ڵ��۵�����
��װ�â��У��ڵ��۵�����![]() �������缫��ӦʽΪ��
�������缫��ӦʽΪ��![]() ��װ�â���ͨ�백����������
��װ�â���ͨ�백����������![]() ��
��
![]() �����Ի����£�NO��
�����Ի����£�NO��![]() ֮��ᷢ��������ԭ��Ӧ����װ�â��з�Ӧ���ɵĺ�������������ʵ�����Ϊ1��1��Ϊ
֮��ᷢ��������ԭ��Ӧ����װ�â��з�Ӧ���ɵĺ�������������ʵ�����Ϊ1��1��Ϊ![]() ��
��![]() ��1��
��1��
![]() ��Һ�еμ���ˮ����������ԭ��Ӧ����Һ��������Ũ�������ƽ�ⳣ��������
��Һ�еμ���ˮ����������ԭ��Ӧ����Һ��������Ũ�������ƽ�ⳣ��������
B.![]() ʱ����Һ�����ԣ�
ʱ����Һ�����ԣ�![]() ����Һ�д��ڵ���غ㣻
����Һ�д��ڵ���غ㣻
C.![]() ʱ��Һ����ҺΪ����������Һ����������غ������
ʱ��Һ����ҺΪ����������Һ����������غ������
D.![]() �ĵڶ������룬
�ĵڶ������룬![]() ��ͼ�з�����֪��
��ͼ�з�����֪��![]() ʱ��
ʱ��![]() ��
��![]() ��
��
E.��ͼ1�����ݣ�![]() ʱʱ���������ƣ�
ʱʱ���������ƣ�![]() ʱ����Һ��Ϊ�������ƣ�����ˮ�ⲻͬ��ˮ�ĵ���̶Ȳ���ͬ��
ʱ����Һ��Ϊ�������ƣ�����ˮ�ⲻͬ��ˮ�ĵ���̶Ȳ���ͬ��
![]() ��
��![]()
��![]()
��![]()
��˹���ɼ��㷴Ӧ![]() ���ʱ䣻
���ʱ䣻
![]() ���Ը��������Һ���������ԣ��������ܣ���Ҫʢ����ʽ�ζ��ܣ���Ӧ�յ��ǵ������һ����Һ���Ϻ�ɫ�жϷ�Ӧ�յ㣻
���Ը��������Һ���������ԣ��������ܣ���Ҫʢ����ʽ�ζ��ܣ���Ӧ�յ��ǵ������һ����Һ���Ϻ�ɫ�жϷ�Ӧ�յ㣻
![]() �ڵ����У������Ϸ�ʧȥ���ӵ�������Ӧ�������Ϸ����õ��ӵĻ�ԭ��Ӧ��
�ڵ����У������Ϸ�ʧȥ���ӵ�������Ӧ�������Ϸ����õ��ӵĻ�ԭ��Ӧ��
![]() ��Ũ��Ϊ
��Ũ��Ϊ![]() ��Ҫʹ
��Ҫʹ![]() ����Һ�е�
����Һ�е�![]() ��ȫת��Ϊ
��ȫת��Ϊ![]() �������ı���������������V����ϵ����غ���м��㡣
�������ı���������������V����ϵ����غ���м��㡣
![]() װ�â���NO������������NO��
װ�â���NO������������NO��![]() ֮��ᷢ��������ԭ��Ӧ����װ�â��з�Ӧ���ɵĺ�������������ʵ�����Ϊ1��1����Ӧ�����ӷ���ʽΪ��
֮��ᷢ��������ԭ��Ӧ����װ�â��з�Ӧ���ɵĺ�������������ʵ�����Ϊ1��1����Ӧ�����ӷ���ʽΪ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
![]() ��Һ�еμ���ˮ����Һ��������Ũ������
��Һ�еμ���ˮ����Һ��������Ũ������ ��
��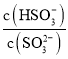 �ı�ֵ���A��ȷ��
�ı�ֵ���A��ȷ��
B��![]() ʱ����Һ�����ԣ�
ʱ����Һ�����ԣ�![]() ����Һ�д��ڵ���غ㣺
����Һ�д��ڵ���غ㣺![]() ������Һ��
������Һ��![]() ����Һ��
����Һ��![]() ����B��ȷ��
����B��ȷ��
C.![]() ʱ��Һ����ҺΪ����������Һ���������غ��֪��
ʱ��Һ����ҺΪ����������Һ���������غ��֪��![]() ����C����
����C����
D.![]() �ĵڶ������룬
�ĵڶ������룬![]() ��ͼ�з�����֪��
��ͼ�з�����֪��![]() ʱ��
ʱ��![]() ��
��![]() ����D��ȷ��
����D��ȷ��
E.��ͼ1�����ݣ�![]() ʱʱ���������ƣ�
ʱʱ���������ƣ�![]() ʱ����Һ��Ϊ�������ƣ�����ˮ�ⲻͬ��ˮ�ĵ���̶Ȳ���ͬ����E����
ʱ����Һ��Ϊ�������ƣ�����ˮ�ⲻͬ��ˮ�ĵ���̶Ȳ���ͬ����E����
BD��ȷ���ʴ�Ϊ��BD��
![]() ��
��![]()
��![]()
��![]()
��˹���ɼ���2��![]() ��
��![]() ��õ���Ӧ�Ȼ�ѧ����ʽΪ��
��õ���Ӧ�Ȼ�ѧ����ʽΪ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
![]() ȡװ�â��е�����ҺVmL����
ȡװ�â��е�����ҺVmL����![]() �����Ը��������Һ�ζ�������Һ��ҺӦװ����ʽ�ζ����У��жϵζ��յ�ķ����ǵ������һ����Һ��Ϊ��ɫ�Ұ���Ӳ���˵����Ӧ�ﵽ�յ㣬�ʴ�Ϊ����ʽ���������һ����Һ��Ϊ��ɫ�Ұ���Ӳ���˵����Ӧ�ﵽ�յ㣻
�����Ը��������Һ�ζ�������Һ��ҺӦװ����ʽ�ζ����У��жϵζ��յ�ķ����ǵ������һ����Һ��Ϊ��ɫ�Ұ���Ӳ���˵����Ӧ�ﵽ�յ㣬�ʴ�Ϊ����ʽ���������һ����Һ��Ϊ��ɫ�Ұ���Ӳ���˵����Ӧ�ﵽ�յ㣻
![]() ���ص����������õ��ӵĻ�ԭ��Ӧ�����Ҳ�缫��ӦʽΪ
���ص����������õ��ӵĻ�ԭ��Ӧ�����Ҳ�缫��ӦʽΪ![]() �������缫��ӦΪ��
�������缫��ӦΪ��![]() ����ͼ��AΪ��Դ��������BΪ��Դ�ĸ�������෴Ӧ���з�������Ҫ�缫��ӦʽΪ
����ͼ��AΪ��Դ��������BΪ��Դ�ĸ�������෴Ӧ���з�������Ҫ�缫��ӦʽΪ![]() ��
��
�ʴ�Ϊ������![]() ��
��
![]() ��Ũ��Ϊ
��Ũ��Ϊ![]() ��Ҫʹ
��Ҫʹ![]() ����Һ�е�
����Һ�е�![]() ��ȫת��Ϊ
��ȫת��Ϊ![]() ����ʧȥ������Ϊ��
����ʧȥ������Ϊ��![]() �������ı���������������V�����ݵ����غ㣺
�������ı���������������V�����ݵ����غ㣺![]() �����
�����![]() ���ù�����ת�Ƶ�����Ŀ
���ù�����ת�Ƶ�����Ŀ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��

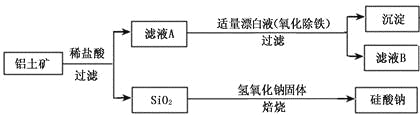
����Ŀ����ͼ�Ǵ���ʿ����Ҫ�ɷ�ΪAl2O3������������SiO2��Fe2O3�����ʣ�����ȡAl2O3������AlN�Ĺ������̣�

��1�����ܽ⡱ʱ����Һ�еĹ�������ƫ�����Ʒ�����Ӧ��2Na2SiO3+2NaAlO2+2H2O��Na2Al2Si2O8��+4NaOH���������Ҫ�ɷ�Ϊ_______��д����ѧʽ����
��2�����ữ��ʱͨ�����CO2��NaAlO2��Ӧ����Al(OH)3����Һ����Ҫ�ɷ�Ϊ______��д����ѧʽ���� ʵ���ҹ������õ��IJ����������ձ���__________����������
��3������ԭ��ʱ��̿���ڸ����±�����ΪCO����Ӧ�Ļ�ѧ����ʽΪ_______��
��4����ȡ���ݲ�ͬ�����ĵ�������Ʒ����������ֻ����̿�ڣ��ֱ�ӵ�20.00 mL��ͬŨ�ȵ�NaOH��Һ�У���ַ�Ӧ���ʵ���������±���ʾ������֪��AlN+NaOH+H2O��NaAlO2+NH3����
ʵ����� | I | II | III |
���뵪������Ʒ������/g | 4.1 | 8.2 | 12.3 |
���ɰ��������/L����״���� | 1.456 | 2.912 | 4.256 |
�ٸ���Ʒ��AlN����������Ϊ_____��
������NaOH��Һ��Ũ��Ϊ___mol/L��
����Ŀ���±������ӷ���ʽ�������۾��������ǣ� ��
ѡ�� | ��ѧ��Ӧ�����ӷ���ʽ | ���� |
A | ������������ϡ����FeO+2H+=Fe2++H2O | �������������ᷴӦ�����κ�ˮ����ȷ |
B | �����������Һ�м���������������ҺBa2++SO | ���ֽⷴӦ���г������ɣ���ȷ |
C | ��nmolFeBr2����Һ��ͨ��nmolCl2��ȫ��Ӧ��2Fe2++2Br-+2Cl2=2Fe3++Br2+4Cl- | ������ԭ��Ӧ���ӷ���ʽ����Ԫ���غ㡢�����غ㡢����غ㣬��ȷ |
D | �Ȼ�����Һ�е������軯����Һ��Fe3++3SCN-=Fe(SCN)3 | ���ֽⷴӦ�����������ɣ��������ɣ�����ȷ |
A.AB.BC.CD.D