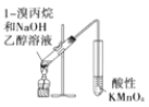
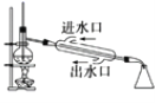
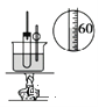
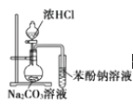
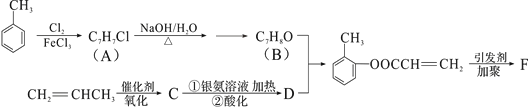
��Ŀ����
����Ŀ����ͼ�Ǵ���ʿ����Ҫ�ɷ�ΪAl2O3������������SiO2��Fe2O3�����ʣ�����ȡAl2O3������AlN�Ĺ������̣�

��1�����ܽ⡱ʱ����Һ�еĹ�������ƫ�����Ʒ�����Ӧ��2Na2SiO3+2NaAlO2+2H2O��Na2Al2Si2O8��+4NaOH���������Ҫ�ɷ�Ϊ_______��д����ѧʽ����
��2�����ữ��ʱͨ�����CO2��NaAlO2��Ӧ����Al(OH)3����Һ����Ҫ�ɷ�Ϊ______��д����ѧʽ���� ʵ���ҹ������õ��IJ����������ձ���__________����������
��3������ԭ��ʱ��̿���ڸ����±�����ΪCO����Ӧ�Ļ�ѧ����ʽΪ_______��
��4����ȡ���ݲ�ͬ�����ĵ�������Ʒ����������ֻ����̿�ڣ��ֱ�ӵ�20.00 mL��ͬŨ�ȵ�NaOH��Һ�У���ַ�Ӧ���ʵ���������±���ʾ������֪��AlN+NaOH+H2O��NaAlO2+NH3����
ʵ����� | I | II | III |
���뵪������Ʒ������/g | 4.1 | 8.2 | 12.3 |
���ɰ��������/L����״���� | 1.456 | 2.912 | 4.256 |
�ٸ���Ʒ��AlN����������Ϊ_____��
������NaOH��Һ��Ũ��Ϊ___mol/L��
���𰸡�Fe2O3��Na2Al2Si2O8 NaHCO3 ©�� ![]() 65% 9.5
65% 9.5
��������
��ʿ��(��Ҫ�ɷ�ΪAl2O3������������SiO2��Fe2O3������)����ʿ���м�������������Һ��SiO2��Al2O3��������������Һ����Һ�еĹ�������ƫ�����Ʒ�����Ӧ:2Na2SiO3+2NaAlO2+2H2O�TNa2Al2Si2O8��+4NaOH������������������������Һ�����Թ��˵ó���ΪFe2O3��Na2Al2Si2O8����Һ��ҪΪƫ��������Һ��ƫ��������Һ��ͨ������Ķ�����̼������������������NaHCO3��Һ�������������յ�����������������̼�������ڸ��������ɵ��������ݴ˴��⡣
��1�����ܽ���ʱ����Һ�еĹ�������ƫ�����Ʒ�����Ӧ��2Na2SiO3+2NaAlO2+2H2O��Na2Al2Si2O8��+4NaOH������������������������Һ�����Թ��˵ó���ΪFe2O3��Na2Al2Si2O8���������Ҫ�ɷ�ΪFe2O3��Na2Al2Si2O8��
��2�����ữ��ʱͨ�����CO2��NaAlO2��Ӧ����Al(OH)3������NaHCO3��������Һ����Ҫ�ɷ�ΪNaHCO3���������õ��IJ����������ձ���©������������
��3������ԭ��ʱ��̿�ڡ���������N,�ڸ��������ɵ�������CO����Ӧ�Ļ�ѧ����ʽΪ![]() ��
��
��4�����������Ʒ�������������ɰ������������˵��ʵ��I����Ʒ��ȫ��Ӧ������������ʣ�࣬��״����1.456L���������ʵ�����![]() ����ʵ��I����Ʒ��AlN������Ϊxg��
����ʵ��I����Ʒ��AlN������Ϊxg��
AlN+NaOH+H2O��NaAlO2+NH3��
41g 1mol
xg 0.065mol
![]() ��x=2.665g��
��x=2.665g��
����Ʒ��AlN����������Ϊ![]() ��
��
ʵ��III��Ʒ��������ʵ��I����Ʒ������3���������ɰ��������С��ʵ��I��3����˵��ʵ��III��Ʒ��ʣ�࣬����������Һ��ȫ��Ӧ����״����4.256 L���������ʵ�����![]() ��
��
AlN+NaOH+H2O��NaAlO2+NH3��
1mol 1mol
xmol 0.19mol
![]() ��x=0.19mol��NaOH��Һ��Ũ��Ϊ
��x=0.19mol��NaOH��Һ��Ũ��Ϊ![]() 9.5mol/L��
9.5mol/L��

 ��У����ϵ�д�
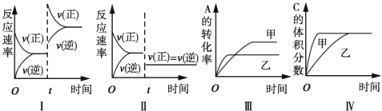
��У����ϵ�д�����Ŀ��ToC�£������Ϊ2 L�ĺ����ܱ�������ͨ��NO2��O2��������Ӧ��4NO2(g)+O2(g)![]() 2N2O5(g)��H��0������ʵ���������±�������˵������ȷ����
2N2O5(g)��H��0������ʵ���������±�������˵������ȷ����
ʱ��/s | 0 | 5 | 10 | 15 |
n(NO2)/mol | 8.00 | n1 | n2 | 4.00 |
n(O2)/mol | 2.00 | 1.25 | 1.00 | n3 |
A. 5s��NO2��ƽ����Ӧ����Ϊ0.3 mol/(Ls��
B. ��10 sʱ�����������г���2 mol N2O5(g)������ƽ���£�NO2���������������
C. ��5 s ʱ�����ھ��Ⱥ����´�ƽ�⣬��ƽ���µ�ƽ�ⳣ����ԭƽ���С
D. ToC���÷�Ӧ��ƽ�ⳣ��Ϊ0.125����Ӧ���ƽ��ת���ʾ�Ϊ50%
����Ŀ�������Ϊ2L�ĺ����ܱ������м���![]() ��һ������CO������Ӧ��
��һ������CO������Ӧ��![]() ��CO��
��CO��![]()
![]() �����ʵ�����ʱ��ı仯���±���ʾ��������˵��������ǣ� ��
�����ʵ�����ʱ��ı仯���±���ʾ��������˵��������ǣ� ��
| 0 | 3 | 10 | 12 |
| 2 | 1 |
|
|
| 0 | 1 |
|
|
A.��![]() �ڣ���
�ڣ���![]() ��ʾ��ƽ����Ӧ����Ϊ
��ʾ��ƽ����Ӧ����Ϊ![]()
B.�ڸ������£�������Ӧ��ƽ�ⳣ��Ϊ3
C.��Ӧ��ƽ��ʱ��![]()
![]() ���������Ϊ
���������Ϊ![]()
D.Ҫ����Ӧ����������![]()
![]() �ڻ�����е�����������ɲ���ѹ�������������ϵѹǿ�Ĵ�ʩ
�ڻ�����е�����������ɲ���ѹ�������������ϵѹǿ�Ĵ�ʩ