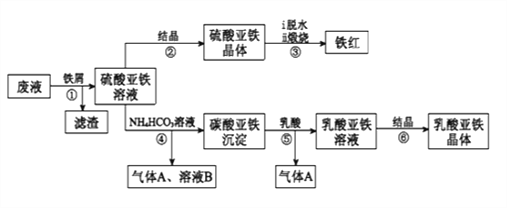
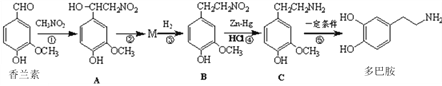
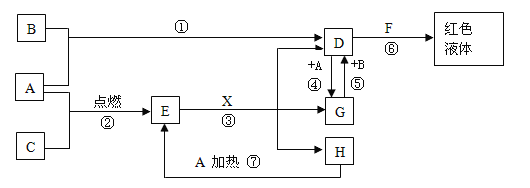
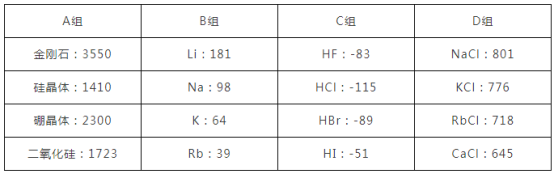
��Ŀ����
����Ŀ������ָ����Ӧ�����ӷ���ʽ��ȷ����
A. ��ʯī�缫���MgCl2��Һ:Mg2++2C1-+2H2O![]() Mg(OH)2��+Cl2��+H2��
Mg(OH)2��+Cl2��+H2��
B. ��������Һ�еμ�̼������Һ:2Al3++3CO32-==Al2(CO3)3��
C. ��Ca(HCO3)2��Һ�еμ�����NaOH��Һ:Ca2++2HCO3-+2OH-==CaCO3��+CO32-+2H2O
D. ��Fe(NO3)3��Һ�м��������HI��Һ:2NO3-+8H++6I-==3I2+2NO��+4H2O
���𰸡�A
��������A. ��ʯī�缫���MgCl2��Һ��Mg2++2Cl-+2H2O![]() Mg(OH)2��+Cl2��+H2����A��ȷ��B. ��������Һ�еμ�̼������Һ����ǿ�ҵ�˫ˮ�⣬�������������Ͷ�����̼���壬B����C. ��Ca(HCO3)2��Һ�еμ�����NaOH��Һ��Ca2++HCO3-+OH-=CaCO3��+H2O��C����D. ��Fe(NO3)3��Һ�м��������HI��Һ�����ᡢ��������������ӷ���������ԭ��Ӧ�������ﻹ�������������ɣ�D����ѡA.
Mg(OH)2��+Cl2��+H2����A��ȷ��B. ��������Һ�еμ�̼������Һ����ǿ�ҵ�˫ˮ�⣬�������������Ͷ�����̼���壬B����C. ��Ca(HCO3)2��Һ�еμ�����NaOH��Һ��Ca2++HCO3-+OH-=CaCO3��+H2O��C����D. ��Fe(NO3)3��Һ�м��������HI��Һ�����ᡢ��������������ӷ���������ԭ��Ӧ�������ﻹ�������������ɣ�D����ѡA.

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ