��Ŀ����
18������a��e����ѧ��ѧʵ���г����ļ��ֶ���������a����Ͳ b������ƿ c���ζ��� d��������ƽ e���¶ȼ�
��ʹ������ƿ�ĵ�һ�������Dz�©��
������������������500mL 2mol•L-1��NaCl��Һ����ȱ�ٵIJ����������ձ�����������ͷ�ιܣ�
���������������ⶨ�к��ȣ���ȱ�ٵIJ����������ձ������β����������
�������������������к͵ζ�����ȱ�ٵIJ�����������ƿ��
���� ��������ƿ��ʹ�÷���������һ�����ʵ���Ũ�ȵ���Һʹ�õ��������к��Ȳⶨʹ���������к͵ζ�����ʹ�õ��������з�����
��� �⣺��ʹ������ƿ�ĵ�һ�������Ǽ������ƿ�Ƿ�©ˮ���ʴ�Ϊ����©��
������������������500mL 2mol•L-1��NaCl��Һ����Ҫ�IJ��������У��ձ�����������500mL����ƿ����ͷ�ιܵȣ���ȱ�ٵIJ��������ǣ��ձ�������������ͷ�ιܣ�
�ʴ�Ϊ������������ͷ�ιܣ�
���������������ⶨ�к��ȣ���ȱ�ٵIJ�������Ϊ���ձ������β�����������ʴ�Ϊ�����β����������
�����������������к͵ζ�����ȱ�ٵ������ǣ���ƿ���ʴ�Ϊ����ƿ��
���� ���⿼���˳���������ʹ�÷�������Ŀ�Ѷ��еȣ������漰�������Դ�֪ʶ��϶࣬��ֿ�����ѧ���������������������Ӧ����ѧ֪ʶ��������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
11�����������У���һ�������¼��ܷ����ӳɷ�Ӧ��Ҳ�ܷ���ȡ����Ӧ��������ʹKMnO4������Һ��ɫ����
��������
��������
| A�� | ���� | B�� | �� | C�� | ��ϩ | D�� | �Ҵ� |
12����NAΪ����٤��������ֵ������˵����ȷ���ǣ�������
| A�� | 31g�����к��еĹ��ۼ���ĿΪ1.5NA | |
| B�� | 0.1L3.0mol•L-1��NH4N03��Һ�к��е�NH4+����ĿΪ0.3NA | |
| C�� | ��״���£�22.4L�����к����ۼ���ĿΪ19NA | |
| D�� | ���³�ѹ�£�lmolCl2��������ˮ��ַ�Ӧ��ת�Ƶĵ�����ĿΪNA |
6�������и���Һ�У�����һ���ܴ���������ǣ�������
| A�� | ������Һ�У�K+��Mg2+��S2-��ClO- | |
| B�� | �����£�pH=1����Һ�У�Na+��Fe3+��NO3-��SO42- | |
| C�� | �������������ݲ�������Һ�У�Na+��NH4+��Fe2+��NO3- | |
| D�� | ��AlCl3��Һ�У�K+��Na+��HCO3-��SO42- |
13���±������еĶ������������ǵ�һ±ȡ�����ֻ��һ�֣������±��и�����Ų����ɣ����˹����Ų���5��ӦΪ��������
| 1 | 2 | 3 | 4 | 5 | �� | �� |
| CH4 | C2H6 | C5H12 | C8H18 | �� | �� | �� |
| A�� | C14H30 | B�� | C17H36 | C�� | C22H42 | D�� | C26H54 |
7���������ӷ�������ȷ���ǣ�������
| A�� | Cu��Ũ���ᷴӦ��Cu+4H++4NO3-�TCu2++4NO2��+2H2O | |
| B�� | ��ˮ�������Һ��Ӧ��NH3•H2O+CH3COOH�TNH4++CH3COO-+H2O | |
| C�� | ������������ˮ��2O2-+2H2O�T4OH-+O2�� | |
| D�� | С�մ�������������Һ��ϣ�HCO3-+OH-�TCO2��+H2O |
8����NA��ʾ�����ӵ�����������˵����ȷ���ǣ�������
| A�� | 18 g H2O�������ĵ�������Ϊ10NA�� | |
| B�� | 1 L 1 mol•L-1�������к��е�HCl������ΪNA�� | |
| C�� | 1 mol I2������ȫ������������������ԼΪ22.4 L | |
| D�� | ������78 g Na2O2�к���O2-����ĿΪ2NA�� |
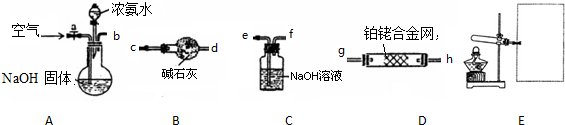
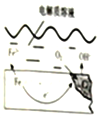 ��ͼ�Ǹ����ڳ�ʪ�����ܷ����绯ѧ��ʴԭ��ʾ��ͼ�������ķ�ӦΪ��2Fe+2H2O+O2�T2Fe��OH��2��Fe��OH��2����������ΪFe��OH��3��Fe��OH��3��ˮ��������
��ͼ�Ǹ����ڳ�ʪ�����ܷ����绯ѧ��ʴԭ��ʾ��ͼ�������ķ�ӦΪ��2Fe+2H2O+O2�T2Fe��OH��2��Fe��OH��2����������ΪFe��OH��3��Fe��OH��3��ˮ��������