��Ŀ����
20�����廯����X�����ֳ����Ķ�����Ԫ����ɣ������������е����Ӽ�����ȡ39.3g������X��������ʵ�飺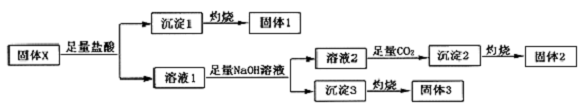
ʵ�������õ�15.3g����2��6.0g����3���ҹ���1������2������3�����������²��ϣ��ش��������⣺
��1��NaOH�ĵ���ʽΪ
 ������2�Ļ�ѧʽΪAl��OH��3��
������2�Ļ�ѧʽΪAl��OH��3����2������X�Ļ�ѧʽΪMgO•Al2O3•2SiO2��
��3����Һ1�м�������NaOH��Һ��Ӧ�����ӷ���ʽΪAl3++4OH-�TAlO2-+2H2O��Mg2++2OH-=Mg��OH��2����
��4���ڸ����£�����3��ijԪ�صĵ��ʿ��������1�����û���Ӧ����д���˷�Ӧ�Ļ�ѧ����ʽ2Mg+SiO2$\frac{\underline{\;����\;}}{\;}$2MgO+Si��
��5�����һ��ʵ�鷽���ȽϹ���2����3�����ֲ�ͬԪ�ض�Ӧ���ʵĻ�����ǿ��ȡ������þ�����ֱ�����������Ũ�ȵ����ᷴӦ����Ӧ���ҵĻ�����ǿ��
���� ����1������2������3�����������²��ϣ���ѧ�г��������²���Ϊ�������衢����þ���������ȣ�����X�����ᷴӦ���ɳ�������ȼ�յù���1�������1ΪSiO2������1ΪH2SiO3������X�����ᷴӦ������Һ1����Һ1�к���þ�������ӣ������������������Ƶ���Һ2�ͳ���3����Һ2����ͨ������̼�ó���2���յù���2������3���յù���3�������2ΪAl2O3������2ΪAl��OH��3������3ΪMgO������3ΪMg��OH��2��39.3g������X�õ�15.3g����2��0.15molAl2O3��6.0g����3��0.15molMgO�����廯����X�����ֳ����Ķ�����Ԫ����ɣ�����Ԫ���غ��֪��X�к���þ�������衢��Ԫ�أ���X�к��й�������������Ϊ39.3g-15.3g-6.0g=18g����X�к���SiO2�����ʵ���Ϊ0.3mol������X�����ΪMgO•Al2O3•2SiO2���ݴ˴��⣮
��� �⣺����1������2������3�����������²��ϣ���ѧ�г��������²���Ϊ�������衢����þ���������ȣ�����X�����ᷴӦ���ɳ�������ȼ�յù���1�������1ΪSiO2������1ΪH2SiO3������X�����ᷴӦ������Һ1����Һ1�к���þ�������ӣ������������������Ƶ���Һ2�ͳ���3����Һ2����ͨ������̼�ó���2���յù���2������3���յù���3�������2ΪAl2O3������2ΪAl��OH��3������3ΪMgO������3ΪMg��OH��2��39.3g������X�õ�15.3g����2��0.15molAl2O3��6.0g����3��0.15molMgO�����廯����X�����ֳ����Ķ�����Ԫ����ɣ�����Ԫ���غ��֪��X�к���þ�������衢��Ԫ�أ���X�к��й�������������Ϊ39.3g-15.3g-6.0g=18g����X�к���SiO2�����ʵ���Ϊ0.3mol������X�����ΪMgO•Al2O3•2SiO2��
��1��NaOHΪ���ӻ�������к������Ӽ����ۼ��������ʽΪ ������2�Ļ�ѧʽΪAl��OH��3��
������2�Ļ�ѧʽΪAl��OH��3��
�ʴ�Ϊ�� ��Al��OH��3��
��Al��OH��3��
��2����������ķ�����֪������X�Ļ�ѧʽΪMgO•Al2O3•2SiO2���ʴ�Ϊ��MgO•Al2O3•2SiO2��
��3����Һ1�м�������NaOH��Һ������������þ��ƫ��������ӣ���Ӧ�����ӷ���ʽΪ Al3++4OH-�TAlO2-+2H2O��Mg2++2OH-=Mg��OH��2����
�ʴ�Ϊ��Al3++4OH-�TAlO2-+2H2O��Mg2++2OH-=Mg��OH��2����
��4������3ΪMgO������1ΪSiO2���ڸ����£�����3��ijԪ�صĵ��ʿ��������1�����û���Ӧ���˷�Ӧ�Ļ�ѧ����ʽΪ2Mg+SiO2$\frac{\underline{\;����\;}}{\;}$2MgO+Si��
�ʴ�Ϊ��2Mg+SiO2$\frac{\underline{\;����\;}}{\;}$2MgO+Si��
��5������2����3�зֱ���þ��������Ԫ�أ��Ƚ�þ�����ֵ��ʵĻ�����ǿ���ķ���Ϊ��ȡ������þ�����ֱ�����������Ũ�ȵ����ᷴӦ����Ӧ���ҵĻ�����ǿ��
�ʴ�Ϊ��ȡ������þ�����ֱ�����������Ũ�ȵ����ᷴӦ����Ӧ���ҵĻ�����ǿ��
���� ���⿼��Ԫ�ػ���������Ժͷ�Ӧ����Ҫ�����˹衢����þ��Ԫ�ؼ��仯�����֪ʶ������ʱע�������ѧ�������ʵ���;���ܸ������ʵ���;�жϻ������ǽⱾ��Ĺؼ�����Ŀ�Ѷ��еȣ�

| A�� | ֻ�������ӻ�����X2Y | |
| B�� | ֻ���ǹ��ۻ�����X2Y2 | |
| C�� | �ȿ��������ӻ�����Ҳ�����ǹ��ۻ����� | |
| D�� | �γɵĻ�����������X2Y����X2Y2 ԭ�Ӷ��ﵽ��8�����ȶ��ṹ |
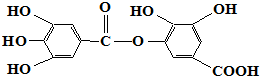
| A�� | �����¿��Ժ�Na2CO3��Һ��Ӧ����CO2���� | |
| B�� | �ڼ���������ˮ�⣬0.5mol��������ȫ��Ӧ��������4mol NaOH | |
| C�� | ��ϡH2SO4�����������ֲ�ͬ���л��� | |
| D�� | �����ʵĻ�ѧʽΪC14H10O9 |
| A�� | pH=14����Һ�У�K+��NH4+��NO3-��HCO3- | |
| B�� | ��ʹ���ȱ�����Һ�У�Na+��K+��SO42-��AlO2- | |
| C�� | 0.1mol•L-1Fe��NO3��2��Һ�У�H+��Ca2+��SCN-��Cl- | |
| D�� | 0.1mol•L-1AlCl3��Һ�У�Cu2+��Mg2+��SO42-��NO3- |
| A�� | ���� | B�� | ������ | C�� | ������ | D�� | ������ |
| A�� | ʵ�����ü�ʯ������SO2 | B�� | CO2�����ʯ��ˮ���� | ||
| C�� | N2O5��ˮ��Ӧ�Ʊ�HNO3 | D�� | Na2O2��ˮ��Ӧ�Ʊ����� |
 ��ѧ��Ӧ�����������ܲ��ɷֵĹ�ϵ���ش��������⣮
��ѧ��Ӧ�����������ܲ��ɷֵĹ�ϵ���ش��������⣮