��Ŀ����
20����ͼΪԪ�����ڱ���һ���֣������Ԫ���ڱ��е�λ�ã��û�ѧ����ش��������⣺
��1������ԭ�Ӱ뾶��С��Ԫ����H ����������ˮ����������ǿ������HClO4 �ڢ�Ԫ����Ӧ���⻯���зе�ϸߵ���SiH4
��2���٢ܢݢ��е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ������ۼ��Ļ����д������һ�ֻ�����ĵ���ʽ
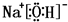 ��
��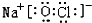 ��
����3���ĵ�����ݵ�����������ˮ�������Һ��Ӧ�����ӷ���ʽ2Al+2OH-+2H2O=AlO2-+3H2��
��4���٢ۢܰ���ԭ�Ӹ�����4��2��3�γ����ӻ�����仯ѧʽΪNH4NO3�������������ӵķ�����ȡ��Ʒ���Թ��У���������������Һ�����ȣ�����ʹʪ��ĺ�ɫʯ����ֽ�����Ĵ̼�����ζ���壬˵������NH4+��
���� ��Ԫ�������ڱ���λ�ã���֪��ΪH����ΪC����ΪN����ΪO����ΪNa����ΪAl����ΪSi����ΪCl��
��1������Ԫ����Hԭ�Ӱ뾶��С����ۺ����������Խǿ�Ǹ�������顢������γɷ��Ӿ��壬��Է�������Խ���Ӽ�������Խǿ���е�Խ�ߣ�
��2���٢ܢݢ��е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ������ۼ��Ļ�������NaOH��NaClO�ȣ�
��3���ĵ���ΪAl���ݵ�����������ˮ����ΪNaOH�����߷�Ӧ����ƫ��������������
��4���٢ۢܰ���ԭ�Ӹ�����4��2��3�γ����ӻ�����ΪNH4NO3��
��� �⣺��Ԫ�������ڱ���λ�ã���֪��ΪH����ΪC����ΪN����ΪO����ΪNa����ΪAl����ΪSi����ΪCl��
��1������Ԫ����Hԭ�Ӱ뾶��С����ۺ����������Խǿ��HClO4�����顢������γɷ��Ӿ��壬��Է�������Խ���Ӽ�������Խǿ���е�Խ�ߣ��ʷе�CH4��SiH4���ʴ�Ϊ��H��HClO4��SiH4��
��2���٢ܢݢ��е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ������ۼ��Ļ�������NaOH��NaClO�ȣ���Ӧ�ĵ���ʽΪ��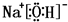 ��
��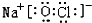 ���ʴ�Ϊ��
���ʴ�Ϊ��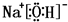 ��
��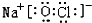 ��
��
��3���ĵ���ΪAl���ݵ�����������ˮ����ΪNaOH�����߷�Ӧ����ƫ����������������Ӧ���ӷ���ʽΪ��2Al+2OH-+2H2O=AlO2-+3H2�����ʴ�Ϊ��2Al+2OH-+2H2O=AlO2-+3H2����
��4���٢ۢܰ���ԭ�Ӹ�����4��2��3�γ����ӻ�����ΪNH4NO3�����к��������ӷ���Ϊ��ȡ��Ʒ���Թ��У���������������Һ�����ȣ�����ʹʪ��ĺ�ɫʯ����ֽ�����Ĵ̼�����ζ���壬˵������NH4+��
�ʴ�Ϊ��NH4NO3��ȡ��Ʒ���Թ��У���������������Һ�����ȣ�����ʹʪ��ĺ�ɫʯ����ֽ�����Ĵ̼�����ζ���壬˵������NH4+��
���� ���⿼��Ԫ�����ڱ���Ԫ�������ɣ��Ƕ�ѧ���ۺ������Ŀ��飬��Ҫѧ���߱���ʵ�Ļ������ѶȲ���

 ��ҵ����ϵ�д�
��ҵ����ϵ�д� ͬ��ѧ��һ�ζ���ϵ�д�
ͬ��ѧ��һ�ζ���ϵ�д� �����ܾ�ϵ�д�
�����ܾ�ϵ�д� ���ƿ�����ϵ�д�
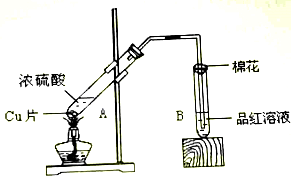
���ƿ�����ϵ�д�| A�� | ���Թ��е�ͭ��Ũ������ȣ������Թܵײ��а�ɫ���岢������������ɫ���ʣ��˰�ɫ����Ϊ����ͭ����ɫ����Ϊ����ͭ | |
| B�� | Ũ�����ڹ��������±�ƣ�˵��Ũ����ȶ������ɵ���ɫ����������Ũ���� | |
| C�� | ��ͬ������ͭ�ֱ��������ĵ������Ũ�����ϡ���ᷴӦ��������Һ�ֱ�Ϊ��ɫ����ɫ�������ڷ�Ӧʱ������ͭ����Ũ��ǰ�ߴ����ں��� | |
| D�� | ��ͭƬ����Ũ�����У�������ʵ������˵��ͭ�����Ũ�����з����ۻ� |
| A�� | ��CH3��2C��Cl��CH2CH3��������2-��-2-������ | |
| B�� | ij��������4��̼ԭ������ͬ���칹����2�֣�������̼ԭ�Ӹ�����ͬ��������4��̼ԭ�ӵĵ�ϩ����4�� | |
| C�� | 1mol�л��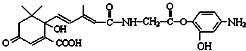 ����NaOH��Һ��Ӧ���������5mol NaOH ����NaOH��Һ��Ӧ���������5mol NaOH | |
| D�� | 1 mol ��-������ͪ �� 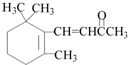 ����1 mol Br2�����ӳɷ�Ӧ�ɵõ�2�ֲ�ͬ���� ����1 mol Br2�����ӳɷ�Ӧ�ɵõ�2�ֲ�ͬ���� |
| A�� | ��ˮ | B�� | ����ͬŨ��FeSO4��Һ������ | ||
| C�� | ����Һ�еμ�ϡ���� | D�� | ��������KMnO4���� |
| A�� | MgΪ��صĸ��� | B�� | ������ӦΪ��AgCl+e-�TAg+Cl- | ||
| C�� | ���ܱ�KCl ��Һ���� | D�� | �����ں���Ӧ���������� |
| A�� | ��ϩʹ������Ȼ�̼��Һ��ɫ | |
| B�� | ����������ˮ�У���ˮ��ӽ���ɫ | |
| C�� | ��ϩʹ���Ը��������Һ��ɫ | |
| D�� | ������������ϣ�����һ��ʱ������ɫ��ʧ |
 ���й����Ҵ��ڸ��ֲ�ͬ��Ӧ�ж��Ѽ���˵���У���ȷ���ǣ�������
���й����Ҵ��ڸ��ֲ�ͬ��Ӧ�ж��Ѽ���˵���У���ȷ���ǣ�������| A�� | �ͽ����Ʒ�Ӧ�����۶��� | B�� | �ͽ����Ʒ�Ӧ�����ڶ��� | ||
| C�� | ��Cu���º�O2��Ӧ�����١��۶��� | D�� | ��Cu���º�O2��Ӧ�����ڡ��۶��� |