��Ŀ����
7��ij��ҵ��ˮ�����±��е�ijЩ���ӣ��Ҹ������ӵ����ʵ���Ũ����ȣ���Ϊ0.1mol/L ������ֵ����ˮ�ĵ��뼰���ӵ�ˮ�⣩| ������ | K+ ��Ag+ ��Mg2+ ��Cu2+ ��Al3+ ��NH4+ |
| ������ | Cl- ��CO32-��NO3 - ��SO42-��I- |
��ȡ����ɫ��Һ5mL���μ�һ�ΰ�ˮ�г������ɣ��������������ӣ�
���ò�˿պȡ��Һ���ڻ��������գ�����ɫ�ܲ����۲죬����ɫ���森
����ȡ��Һ����������ᣬ����ɫ�������ɣ�����ɫ������������ɺ���ɫ��
������������õ���Һ�м���BaCl2 ��Һ���а�ɫ�������ɣ�
���ƶϣ�
��1���ɢ��жϣ���Һ��һ�������е���������K+��NH4+��Cu2+��
��2�����м�������������ɫ��������ӷ���ʽ��6I-+2NO3-+8H+�T3I2+2NO��+4H2O��
��3����ͬѧ����ȷ��ԭ��Һ��������������Mg2+��Al3+����������Cl-��I-��NO3-��SO42-�����ݴ��Ʋ�ԭ��ҺӦ�ó����ԣ�ԭ����Mg2++2H2O?Mg��OH��2+2H+��Al3++3H2O?Al��OH��3+3H+�������ӷ���ʽ˵������
��4����ȡ100mLԭ��Һ������������NaOH��Һ���˹������漰�����ӷ���ʽΪMg2++2OH-�TMg��OH��2����Al3++4OH-�TAlO2-+2H2O����ַ�Ӧ����ˣ�ϴ�ӣ����ճ��������أ��õ��Ĺ�������Ϊ0.4g��
���� ��ȡ����ɫ��Һ5mL��˵��һ��������Cu2+���μ�һ�ΰ�ˮ�г������ɣ��������������ӣ�˵�����ӵ���NH4+������ԭ��Һ��һ������NH4+�����ܺ���Mg2+��Al3+������NH4+��CO32-��
���ò�˿պȡ������Һ���ڻ��������գ�����ɫ���棨����ɫ�ܲ����۲죩��˵��û��K+��
����ȡ��Һ�����������ᣬ����ɫ�������ɣ�����ɫ������������ɺ���ɫ����ʱ��Һ��Ȼ���壬˵���л�ԭ������ I-��NO3-��H+��Ӧ����NO������Һ����I-��NO3-�����ж�һ��������Ag+��
������������õ���Һ�м���BaCl2��Һ���а�ɫ�������ɣ�˵����SO42-�����������жϷ�����
��� �⣺��ȡ����ɫ��Һ5mL��˵��һ��������Cu2+���μ�һ�ΰ�ˮ�г������ɣ��������������ӣ�˵�����ӵ���NH4+������ԭ��Һ��һ������NH4+�����ܺ���Mg2+��Al3+������NH4+��CO32-��
���ò�˿պȡ������Һ���ڻ��������գ�����ɫ���棨����ɫ�ܲ����۲죩��˵��û��K+��
����ȡ��Һ�����������ᣬ����ɫ�������ɣ�����ɫ������������ɺ���ɫ����ʱ��Һ��Ȼ���壬˵���л�ԭ������ I-��NO3-��H+��Ӧ����NO������Һ����I-��NO3-�����ж�һ��������Ag+��
������������õ���Һ�м���BaCl2��Һ���а�ɫ�������ɣ�˵����SO42-��
��1���ɢ��жϣ���Һ��һ�������е���������K+��NH4+��Cu2+��
�ʴ�Ϊ��K+��NH4+��Cu2+��
��2�����м�����������������ɫ���壬��I-��NO3-��H+��Ӧ����NO�������ӷ���ʽ��6I-+2NO3-+8H+�T3I2+2NO��+4H2O��
�ʴ�Ϊ��6I-+2NO3-+8H+�T3I2+2NO��+4H2O��
��3��������������֪һ�����е�������I-��NO3-��SO42-���Ҹ�Ϊ0.1mol/L�����ݵ���غ��֪���ƶϳ��������Ӻ���Mg2+��Al3+����Ũ��Ϊ0.1mol/L���ɵ���غ��֪��Һ�л���һ��-1�۵�������ΪCl-�����Լ�ͬѧ����ȷ��ԭ��Һ�������������ǣ�Mg2+��Al3+���������ǣ�Cl-��I-��NO3-��SO42-����Һ��þ���Ӻ�������ˮ����Һ�����ԣ���Ӧ�����ӷ���ʽΪ��Mg2++2H2O?Mg��OH��2+2H+��Al3++3H2O?Al��OH��3+3H+��
�ʴ�Ϊ��Mg2+��Al3+��Cl-��I-��NO3-��SO42-���Mg2++2H2O?Mg��OH��2+2H+��Al3++3H2O?Al��OH��3+3H+��
��4����ȡ100mLԭ��Һ������������NaOH��Һ��Mg2+��Al3+ ��Ӧ����Mg��OH��2��NaAlO2���漰�����ӷ���ʽΪMg2++2OH-�TMg��OH��2����Al3++4OH-�TAlO2-+2H2O����ַ�Ӧ����ˣ�ϴ�ӣ����ճ��������أ�����Ԫ���غ㣺n��MgO��=n��Mg2+��=cV=0.1mol/L��0.1L=0.01mol��m��MgO��=0.01mol��40g/mol=0.4g��
�ʴ�Ϊ��Mg2++2OH-�TMg��OH��2����Al3++4OH-�TAlO2-+2H2O��0.4��
���� ���⿼�����ʵļ��顢�����Լ�����ʽ���йؼ��㣬��Ŀ�Ѷ��еȣ������Ĺؼ��ǰ����йط�Ӧ�Ļ�ѧ����ʽ����д��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�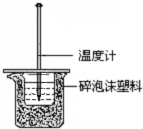 ijͬѧ��40mL 0.50mol/L�����40mL 0.55mol/L NaOH
ijͬѧ��40mL 0.50mol/L�����40mL 0.55mol/L NaOH��Һ��Ӧ���ⶨǿ����ǿ�Ӧ�ķ�Ӧ�ȣ�װ����ͼ��ʾ����ش��������⣮
��1��ͼʾʵ��װ����ȱ�ٵ�һ�������ǻ��β������������С�ձ���������ĭ���ϵ������Ǽ���ʵ������е�������ʧ��
��2�������ձ��ϲ���Ӳֽ�壬��õġ�H��ƫ���ƫ����ƫС������Ӱ�족����
��3����ͬѧʵ�����ݼ�¼���£�
| �¶�ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�t2/�� | |
| ���� | NaOH��Һ | �����Һ | |
| 1 | 20.0 | 20.1 | 23.2 |
| 2 | 20.2 | 20.4 | 23.4 |
| 3 | 20.5 | 20.6 | 23.6 |
| A�� | Cl2 | B�� | N2 | C�� | H2 | D�� | CO2 |
| A�� | 0.44 g | B�� | 0.88 g | C�� | 0.66 g | D�� | ������ |
| A�� | ��NH4��2SO4����ˮ��NH4��2SO4�T2NH4++SO42- | |
| B�� | H3PO4����ˮH3PO4?3H++PO43- | |
| C�� | HF����ˮHF?H++F | |
| D�� | NaHS����ˮNaHS�TNa++HS-��HS-?H++S2- |
| A�� | ���ʶ�����Ԫ����ɵ� | |
| B�� | ����Ԫ��ֻ���γ�һ�ֻ����� | |
| C�� | ���ʵķ����ж������������ֻ��һ�ַ��������������������� | |
| D�� | ���������������Ӧ�����κ�ˮ | |
| E�� | ���������������ˮ��Ӧ������Ӧ���� | |
| F�� | ���������������ˮ��Ӧ������Ӧ�ļ� |
���������м������ϡ���ᣬ���ˣ�
������Һ�м������KMnO4��Һ��Ȼ�������Һ��PHԼΪ3��
���ȣ�����������ɫ���������ã��ϲ���Һ���Ϻ�ɫ��
��������MnSO4���Ϻ�ɫ��ʧ�����ˣ�
����Ũ�����ᾧ�����룬�õ���Ʒ��
��֪�������������������PH�Ĺ�ϵ
| Al��OH��3 | Fe��OH��2 | Fe��OH��3 | |
| ��ʼ����ʱ | 3.4 | 6.3 | 1.5 |
| ��ȫ����ʱ | 4.7 | 8.3 | 2.8 |
��2����֪��һ�������£�MnO4-��Mn2+��Ӧ����MnO2��
�����������ij����м���Ũ���Ტ���ȣ���˵�������д���MnO2�����������ɻ���ɫ���壮
�ڢ��м���MnSO4��Ŀ���dz�ȥ������MnO4-��
��3����Al2��SO4��3�ֲ�Ʒ���ж���������������ͼ��ʾ��
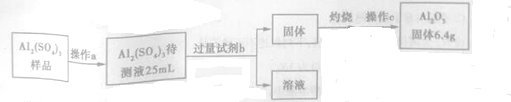
�ٲ���c�dz�����������������Ϊ������ƽ��
�ڼ����Լ�b������Ӧ�����ӷ���ʽΪAl3++3NH3•H2O�TAl��OH��3��+3NH4+��
�����Ƶ�Al2��SO4��3�Ĵ���Һ��c��Al3+��=5.0mol/L����������λ��Ч���֣�
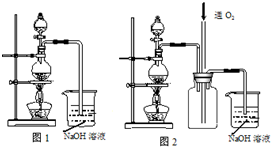 ����ͭ��һ��Ӧ�ü���㷺�Ļ���ԭ�ϣ�ʵ���пɽ�����Ũ����ֶ�μ��뵽ͭ����ϡ����Ļ�����У�����ʹ֮��Ӧ��ȫ��ͨ���������ᾧ�õ�����ͭ���壨װ����ͼ1��ʾ����
����ͭ��һ��Ӧ�ü���㷺�Ļ���ԭ�ϣ�ʵ���пɽ�����Ũ����ֶ�μ��뵽ͭ����ϡ����Ļ�����У�����ʹ֮��Ӧ��ȫ��ͨ���������ᾧ�õ�����ͭ���壨װ����ͼ1��ʾ����