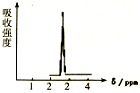
��Ŀ����
4�������ǵ���������������������в�������������ȴ�ӹ���IJ�������óɷ�Ϊ���������������������������輰�����������������������ȣ��������Ʊ�Al2��SO4��3•18H2O�������������£����ֲ����������ԣ������������м������ϡ���ᣬ���ˣ�
������Һ�м������KMnO4��Һ��Ȼ�������Һ��PHԼΪ3��
���ȣ�����������ɫ���������ã��ϲ���Һ���Ϻ�ɫ��
��������MnSO4���Ϻ�ɫ��ʧ�����ˣ�
����Ũ�����ᾧ�����룬�õ���Ʒ��
��֪�������������������PH�Ĺ�ϵ
| Al��OH��3 | Fe��OH��2 | Fe��OH��3 | |
| ��ʼ����ʱ | 3.4 | 6.3 | 1.5 |
| ��ȫ����ʱ | 4.7 | 8.3 | 2.8 |
��2����֪��һ�������£�MnO4-��Mn2+��Ӧ����MnO2��
�����������ij����м���Ũ���Ტ���ȣ���˵�������д���MnO2�����������ɻ���ɫ���壮
�ڢ��м���MnSO4��Ŀ���dz�ȥ������MnO4-��
��3����Al2��SO4��3�ֲ�Ʒ���ж���������������ͼ��ʾ��
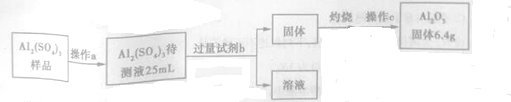
�ٲ���c�dz�����������������Ϊ������ƽ��
�ڼ����Լ�b������Ӧ�����ӷ���ʽΪAl3++3NH3•H2O�TAl��OH��3��+3NH4+��
�����Ƶ�Al2��SO4��3�Ĵ���Һ��c��Al3+��=5.0mol/L����������λ��Ч���֣�
���� ���Ҽ������ϡ�������ܹ��ˣ���ȥ����������SiO2���õ���Һ�к���Al3+��Fe3+��Fe2+��������������Һ������ҺPH������������Ϊ�����ӣ�����Һ�м������KMnO4��Һ��Ŀ����������������Ϊ���������ӣ�����ͼ�����ݷ�����֪�������ӿ�ʼ�����ͳ�����ȫ����ҺpHΪ1.5��2.8�������Ӻ��������ӿ�ʼ��������ҺpH����3�����Ե�����Һ��pHԼΪ3������ʹ������ȫ�������������Ӳ��������룻���ij����м���ŨHCl�����ȣ��������̺�Ũ�����ڼ��������·�Ӧ�����Ȼ��̡�������ˮ�����ɵ������ǻ���ɫ���壬����MnSO4�����ĸ��������Һ��Ӧ���ɶ������̳��������˵õ���Һ����Ҫ�������ӣ�����Ũ�����ᾧ�����룬�õ���Ʒ��
��1��MnO4-����Fe2+��MnԪ�صĻ��ϼ���+7�۽���Ϊ+2�ۣ�FeԪ�صĻ��ϼ���+2������Ϊ+3�ۣ���ϵ��ӡ�����غ㼰ԭ���غ������
��2����Ũ����Ͷ��������ټ������������ɻ���ɫ����������
�ڼ���MnSO4���Ϻ�ɫ��ʧ��Ŀ���dz�ȥ��������������ӣ�
��3��Al2��SO4��3�ֲ�Ʒ���ж����������Ƚ��ֲ�Ʒ����ˮ�����˳�ȥ�����ȡ25mL������Һ�����백ˮ���������������������˵õ������������壬���պ���Ϊ���壬����������������ƽ���ݴ˷������
��� �⣺��1��MnO4-����Fe2+��MnԪ�صĻ��ϼ���+7�۽���Ϊ+2�ۣ�FeԪ�صĻ��ϼ���+2������Ϊ+3�ۣ��ɵ��ӡ�����غ㼰ԭ���غ��֪����ӦΪMnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O��
�ʴ�Ϊ��MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O��
��2�������ij����м���ŨHCl�����ȣ��������̺�Ũ�����ڼ��������·�Ӧ�����Ȼ��̡�������ˮ�����ɵ������ǻ���ɫ���壬��˵�������д���MnO2�����������ɻ���ɫ���壻
�ʴ�Ϊ�����ɻ���ɫ���壻
��һ�������£�MnO4-����Mn2+��Ӧ����MnO2�����˳�ȥ�����Կ�������MnSO4����Һ���������Һ��Ӧ���ɶ������̣��ѹ�������������ӳ�ȥ��
�ʴ�Ϊ����ȥ������MnO4-��
��3��Al2��SO4��3�ֲ�Ʒ���ж����������Ƚ��ֲ�Ʒ����ˮ�����˳�ȥ�����ȡ25mL������Һ�����백ˮAl3++3NH3•H2O�TAl��OH��3��+3NH4+�����˵õ������������壬����2Al��OH��3$\frac{\underline{\;\;��\;\;}}{\;}$Al2O3+3H2O������Al2O3������������ƽ��6.4gAl2O3�����ʵ���Ϊ��n=$\frac{6.4g}{102g/mol}$��0.06275mol��n[Al2O3]=n[Al2��SO4��3]=0.06275mol�����Ƶ�Al2��SO4��3�Ĵ���Һ��c��Al3+��=$\frac{0.06275mol��2}{0.025L}$��5.0mol/L��
�ʴ�Ϊ��������������ƽ��Al3++3NH3•H2O�TAl��OH��3��+3NH4+��5��
���� ���⿼����þ�����������仯�������ʵ�Ӧ�ã���Ҫ�ǻ�������ķ�����ʵ����ƣ����������������������ӣ�������ҺPH�dz��������ǽ���Ĺؼ�����Ŀ�Ѷ��еȣ�

| ������ | K+ ��Ag+ ��Mg2+ ��Cu2+ ��Al3+ ��NH4+ |
| ������ | Cl- ��CO32-��NO3 - ��SO42-��I- |
��ȡ����ɫ��Һ5mL���μ�һ�ΰ�ˮ�г������ɣ��������������ӣ�
���ò�˿պȡ��Һ���ڻ��������գ�����ɫ�ܲ����۲죬����ɫ���森
����ȡ��Һ����������ᣬ����ɫ�������ɣ�����ɫ������������ɺ���ɫ��
������������õ���Һ�м���BaCl2 ��Һ���а�ɫ�������ɣ�
���ƶϣ�
��1���ɢ��жϣ���Һ��һ�������е���������K+��NH4+��Cu2+��
��2�����м�������������ɫ��������ӷ���ʽ��6I-+2NO3-+8H+�T3I2+2NO��+4H2O��
��3����ͬѧ����ȷ��ԭ��Һ��������������Mg2+��Al3+����������Cl-��I-��NO3-��SO42-�����ݴ��Ʋ�ԭ��ҺӦ�ó����ԣ�ԭ����Mg2++2H2O?Mg��OH��2+2H+��Al3++3H2O?Al��OH��3+3H+�������ӷ���ʽ˵������
��4����ȡ100mLԭ��Һ������������NaOH��Һ���˹������漰�����ӷ���ʽΪMg2++2OH-�TMg��OH��2����Al3++4OH-�TAlO2-+2H2O����ַ�Ӧ����ˣ�ϴ�ӣ����ճ��������أ��õ��Ĺ�������Ϊ0.4g��
| A�� |  | |
| B�� | Һ�ȡ����� | |
| C�� | O2��O3 | |
| D�� | ${\;}_{17}^{35}Cl$��${\;}_{17}^{37}Cl$ |
| A�� | ��ȼ���ͷŵļ��������� | B�� | ��ȼ����ˮ���ͣ����ڻ�ѧ�仯 | ||
| C�� | �������춡��Ϊͬϵ�� | D�� | ��Ȼ����һ����Ҫ�Ļ���ԭ�� |
| A�� | �ں���Fe3+��������Һ�У�NH4+��Na+��Cl-��SCN- | |
| B�� | ij��ɫ����Һ��NH4+��K+��SO32-��Cr2O72- | |
| C�� | ��c��H+��=0.1mol/L����Һ�У�K+��Mg2+��Cl-��NO3- | |
| D�� | ������Al��Ӧ����H2����Һ�У�NH4+��NO3-��SO42-��HCO3- |
| A�� | ����ͨ��������ǹ��� | |
| B�� | ���Դ���-3��+3��+5�ȶ��ֻ����� | |
| C�� | As2O5��Ӧˮ��������Ա�H3PO4ǿ | |
| D�� | �����̬�⻯�������̬�⻯���ȶ��Բ� |
| A�� | �������ϩ������������һ�������·����ӳɷ�Ӧ | |
| B�� | �Ҵ����������������������Һ��Ӧ�����κ�ˮ | |
| C�� | �߷��ӻ�������ָ��Է�����������1000�Ļ����� | |
| D�� | �Ҵ�����������������ñ���Na2CO3��Һ���� |
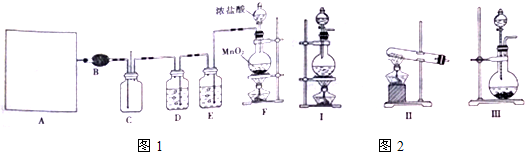
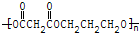 ��
��