��Ŀ����
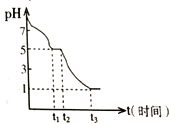
����Ŀ����������������Ԫ�أ���ҽҩ��ҵ�ж��й㷺��;����˴Ӻ����Һ�л��յ������ö�����Դ�Ƿdz���Ҫ�ġ�ʵ���ҴӺ����Һ(��H2O�⣬����CCl4��I2��I-���л��յ⣬��ʵ�������ͼ1������֪������Cl2> IO3-��
��1�����Һ�м����Թ�����Na2SO3��Һ����Ӧ�����ӷ���ʽΪ_________���ò�����I2��ԭΪI-��Ŀ����_________________________________________��
��2������X������Ϊ______________________________________��
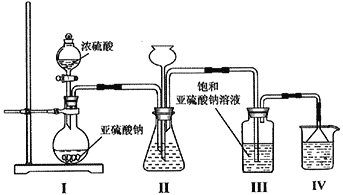
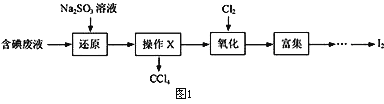
��3������ʱ����������ƿ�н���I-��ˮ��Һ���������pHԼΪ2��ͨ��Cl2����40�����ҷ�Ӧ��ʵ��װ����ͼ2��ʾ���������й�˵����ȷ����_____������ĸ����
a.��ʵ����Ҫ����ͨ����������������������ʣ���ֹ������Ⱦ
b.�����ڽϵ��¶��½��е���Ҫԭ���������������ܽ��
c.ͨ���������������ߵ�IJ���
d.Ϊ��Ч��ֹ��Ļӷ�ˮӦ��b��ͨ��
e.��ƿ��Ӧʢ��NaOH��Һ
��4���������ȣ�C1O2������ɫ������ˮ�����壩�Ǹ�Ч���Ͷ�����������ˮ������������C1O2�������Ժ�I-��Һ���յ⡣
��д��C1O2����I-�����ӷ���ʽ____________________________________��
����������I-��ͬ���ķ�Һ���յ⡢����Cl2�����ʵ�����C1O2��______________����
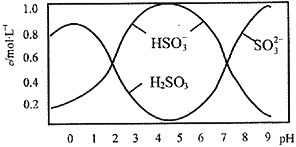
��5����֪��5SO32-+2IO3-+2H+![]() I2+5SO42-+H2O��ij�����ˮ��pHԼΪ8����һ������I2�����ܴ���I-��IO3-�е�һ�ֻ����֡��벹���������麬���ˮ���Ƿ���I-��IO3-��ʵ�鷽������ʵ���пɹ�ѡ����Լ���ϡ���ᡢ������Һ��FeCl3��Һ��Na2SO3��Һ��
I2+5SO42-+H2O��ij�����ˮ��pHԼΪ8����һ������I2�����ܴ���I-��IO3-�е�һ�ֻ����֡��벹���������麬���ˮ���Ƿ���I-��IO3-��ʵ�鷽������ʵ���пɹ�ѡ����Լ���ϡ���ᡢ������Һ��FeCl3��Һ��Na2SO3��Һ��
��ȡ���������ˮ��CCl4�����ȡ����Һ��ֱ��ˮ����鲻���ⵥ�ʴ��ڣ�
��_______________________��
������ˮ��ȡ������Һ������2-3�ε�����Һ���μ�________________�����Լ�������˵����ˮ�к���IO3-������˵����ˮ�в���IO3-��
���𰸡� H2O+SO32-+I2=2I-+SO42-+2H+ ʹCCl4�еĵ����ˮ�㣨��I2ת��ΪI-��CCl4�з�������� ��Һ abe 2C1O2+10I-+8H+=2Cl-+5I2+4H2O 2.5 ��ˮ����ȡ������Һ������2~3�ε�����Һ����ϡ�����ữ���μ�FeCl3��Һ������Һ��������֤����ˮ�д���I-������˵����ˮ�в���I- ����ϡ���������������Һ������Һ����
��������I�������Һ(��H2O�⣬����CCl4��I2��I-)�м�����������Һ���ѵ��ʵԭΪI-�����Ȼ�̼������ˮ����ֲ㣬���Һ���ɵõ����Ȼ�̼��ʣ�����Һ�м���������I-�õ�I2�����������õ��ϴ���I2��
(1)����������ԣ������������������������ƣ���������ԭ���ɵ����ӣ����ӷ�Ӧ����ʽΪSO32-+I2+H2O=2I-+2H++SO42-����������ˮ����������������ˮ��Ϊ��ʹ�����IԪ�ؽ���ˮ��ҺӦ���ԭΪ�����ӣ��ʴ�Ϊ��SO32-+I2+H2O=2I-+2H++SO42-��ʹCCl4�еĵ����ˮ��(��I2ת��ΪI-��CCl4�з������)��
(2)���Ȼ�̼������ˮ������ֲ㣬���뻥�����ܵ�Һ����÷�Һ�ķ������룬���Է�������Ȼ�̼���÷�Һ�ķ������ʴ�Ϊ����Һ��
(3)a.��ʵ����Ҫ����ͨ��������ʹ������ַ�Ӧ��������������������ʣ���ֹ������Ⱦ����ȷ��b.������ܽ�����¶ȵ����߶����ͣ������ڽϵ��¶��½��п��������������ܽ�ȣ���ȷ��c.ͨ���������������֤��������ȫ���������������������ܹ���������������ɺ���Ԫ�ص������ӣ��������͵�IJ���������d.��ȴˮӦ����ѭ�½��ϳ���Ϊ��Ч��ֹ��Ļӷ�ˮӦ��a��ͨ�룬����e.Ϊ�˷�ֹ������Ⱦ��������ƿ��Ӧʢ��NaOH��Һ��������������������ȷ����ѡabe��
(4)����ClO2�������Ժ�I-��Һ���յ⣬�Ƕ�������������Һ���������������ɵⵥ�ʣ��������ȱ���ԭΪ�����ӣ�ClO2��Cl-��5e-��2I-��I2��2e-����Ӧ�����ӷ���ʽΪ2ClO2+10I-+8H+=5I2+2Cl-+4H2O���ʴ�Ϊ��2ClO2+10I-+8H+=5I2+2Cl-+4H2O��
����������ԭ��Ӧ�����غ㣬ÿĦ��Cl2�õ�2mol���ӣ���ÿĦ��ClO2�õ�5mol���ӣ�������Cl2�����ʵ�����ClO2��2.5�����ʴ�Ϊ��2.5��
(5)�ڵ����Ӿ��л�ԭ�ԣ��ܱ��������������ɵ⣬��������Ӿ��������ԣ��ܱ���ԭ����ԭ���ɵ⣬����������Һ����ɫ����������鷽��Ϊ����ˮ��ȡ������Һ������1-2mL������Һ�����������ữ���μ�FeCl3��Һ��2I-+2Fe3+=2Fe2++I2������Һ����ɫ��˵����ˮ�к���I-������I-���ʴ�Ϊ����ˮ��ȡ������Һ������1-2mL������Һ�����������ữ���μ�FeCl3��Һ������Һ����ɫ��˵����ˮ�к���I-������I-��
������ˮ��ȡ������Һ������2-3�ε�����Һ���μ�����ϡ���������������Һ������Һ������˵����ˮ�к���IO3-������˵����ˮ�в���IO3-���ʴ�Ϊ������ϡ���������������Һ������Һ������