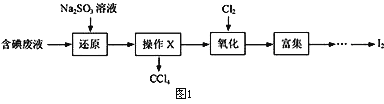
��Ŀ����
����Ŀ����֪Ǧ���صĹ���ԭ��Ϊ��Pb��PbO2��2H2SO4![]() 2PbSO4��2H2O��������ͼװ�ý��е��(���Һ����)����õ�Ǧ������ת��0.4 mol����ʱ���缫����������11.2 g����ش��������⡣
2PbSO4��2H2O��������ͼװ�ý��е��(���Һ����)����õ�Ǧ������ת��0.4 mol����ʱ���缫����������11.2 g����ش��������⡣

��1��A��Ǧ���ص�________����Cu�缫��________�����ŵ�����е��Һ���ܶ�________(������С��������������������)��
��2��Ag�缫�ĵ缫��Ӧʽ��______________________________���õ缫�ĵ缫���ﹲ________g��
��3��Cu�缫�ĵ缫��Ӧʽ��______________________________��CuSO4��Һ��Ũ��________(������С��������������������)
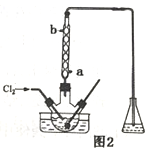
��4����ͼ��ʾ�����й�����ij����(������x)��ʱ��ı仯���ߣ���x��ʾ________��

a����U�ι��в�������������
b����U�ι������������ļ�����
c����U�������������������
���𰸡� �� �� ��С 2H����2e��===H2�� 0.4 Cu��2e��===Cu2�� ���� b
����������1����Ǧ������ת��0.4mol����ʱ���缫��������С11.2g��˵������������������������������ԭ��ظ���������A�Ǹ�����B�������������϶�����Ǧ�õ��ӷ�����ԭ��Ӧ���缫��ӦʽΪPbO2+4H��+SO42��+2e���TPbSO4+2H2O�����ݵ�ⷽ��ʽ�жϣ���������pHֵ�������ᱻ��Ӧ�����ŵ�����е��Һ���ܶȱ�С����2���������������ϡ����ʱ�������������ӷŵ������������缫��ӦʽΪ2H��+2e���TH2������������������=0.4mol/2��2g��mol��1=0.4g����3��Cu�缫�ĵ缫��Ӧʽ��Cu��2e��=Cu2����Zn�缫�ϵķ�Ӧ��Cu2��+2e��=Cu��Cu�缫�ܽ��ͭ��Zn�缫������ͭ�����CuSO4��Һ��Ũ�Ȳ�������4��a���ұ�U�ιܲ��������壬���U�ι��������壬����ϡ�����������������������ͭ��Һ���ʴ���b����ת����ȵ���ʱ���ܽ���������ʵ�����ȣ�ͭ��Ħ�������������������ұ�U�ι��������ٵ������������U�ι��������ٵ�����������ȷ��c����ת����ȵ���ʱ���������ʵ����ʵ�����ȣ���ͭ��Ħ�����������������������U�ι���������������С���ұ�U�ι�����ͭ���������ʴ���ѡb��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ������ˮ��ɽ���ǽ�ɽ��ɽ�����о�NO2��NO��CO��S02�ȴ�����Ⱦ���ˮ��Ⱦ��Ĵ����Խ��������й�������Ҫ���塣
(1)��֪�� ��NO2+CO![]() CO2+NO�÷�Ӧ��ƽ�ⳣ��ΪK1(��ͬ)��ÿ1mol�������ʷֽ�Ϊ��̬��̬ԭ�����յ������ֱ�Ϊ
CO2+NO�÷�Ӧ��ƽ�ⳣ��ΪK1(��ͬ)��ÿ1mol�������ʷֽ�Ϊ��̬��̬ԭ�����յ������ֱ�Ϊ
NO2 | CO | CO2 | NO |
812kJ | 1076kJ | 1490kJ | 632kJ |
��N2(g)+O2(g) ![]() 2NO(g) ��H=+179.5kJ/mol K2
2NO(g) ��H=+179.5kJ/mol K2
��2NO(g)+O2(g)![]() 2NO2(g) ��H=-112.3kJ/mol K3
2NO2(g) ��H=-112.3kJ/mol K3
д��NO��CO��Ӧ��������Ⱦ������Ȼ�ѧ����ʽ____________________________________�����Ȼ�ѧ����ʽ��ƽ�ⳣ��K=_________(��K1��K2��K3��ʾ)��
(2)������ɱ�ĺ�ѹ(p��)�ܱ������г���1molCO2 ��������̼�����䷢����Ӧ�� C(s)+ CO2(g)![]() 2CO(g) ��H>0��ƽ��ʱ����ϵ����������������¶ȵĹ�ϵ����ͼ��ʾ��
2CO(g) ��H>0��ƽ��ʱ����ϵ����������������¶ȵĹ�ϵ����ͼ��ʾ��
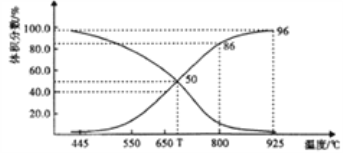
��T��ʱ����������������ϡ�����壬v(��)___v(��)(�>����<����=")��ƽ��______�ƶ�(���������������ͬ)��������������CO2 ��CO��ƽ��________�ƶ���
��CO�������Ϊ40%ʱ��CO2 ��ת����Ϊ_______��
����֪�������ѹ(p��)=������ѹ�������������ƽ���ѹ����ƽ��Ũ�ȱ�ʾƽ��Kp�����ı���ʽΪ__________��925��ʱ��Kp=______(�ú�p���Ĵ���ʽ��ʾ)��
(3)ֱ���ŷź�SO2 ���������γ����꣬Σ������������NaOH���գ����ú������(H2SO3��HSO3-��SO32-)�����ڷ�Ӧ�����Һ�У����ǵ����ʵ�������X(i)����ҺpH�Ĺ�ϵ��ͼ��ʾ��

������0.1mol/LNaOH ��Ӧ�����Һ�������Һ��pH=8ʱ����Һ�и�����Ũ���ɴ�С��˳����______________��
����pH=5��NaHSO3��Һ�еμ�һ��Ũ�ȵ�CaCl2 ��Һ����Һ�г��ֻ��ǣ�pH��Ϊ2���û�ѧƽ���ƶ�ԭ��������ҺpH���͵�ԭ��_______________________________________________��