��Ŀ����
9����������㷺��������Ȼ���У�ɳ�ӡ�ʯӢ����Ҫ�ɷ־��Ƕ������裮��1����ҵ�����ô��ʯ��ʯ��ʯӢ�����Ʊ���ͨ�������漰�Ļ�ѧ����ʽΪNa2CO3+SiO2$\frac{\underline{\;����\;}}{\;}$Na2SiO3+CO2����CaCO3+SiO2CaSiO3+CO2����
��2�����NaOH�ȼ�����Һ�IJ����Լ�ƿ������ĥɰ��������ԭ����SiO2+2NaOH=
Na2SiO3+H2O���û�ѧ����ʽ��ʾ����
��3�����ټ�����������Ź㷺Ӧ�ã�����Ȼ���в���������̬�Ĺ裮��ҵ�����ö��������Ƶôֹ裬�÷�Ӧ�Ļ�ѧ����ʽΪSiO2+2C$\frac{\underline{\;����\;}}{\;}$Si+2CO����
���� ��1������������ԭ���Ǵ��ʯӢ�ʹ���ʯ��̼��ƿ��ԺͶ�������֮�䷢����Ӧ�����ɹ���ƺͶ�����̼��
��2�����ݲ����ijɷּ��������ơ�������������ʷ�����
��3�����ټ�����������Ź㷺Ӧ�ã�����Ȼ���в���������̬�Ĺ裬��ҵ�����ö��������Ƶôֹ裮
��� �⣺��1������������ԭ���Ǵ��ʯӢ�ʹ���ʯ��̼��ƿ��ԺͶ�������֮�䷢����Ӧ���ɹ���ƺͶ�����̼����CaCO3+SiO2$\frac{\underline{\;����\;}}{\;}$CaSiO3+CO2�����ʴ�Ϊ��Na2CO3+SiO2$\frac{\underline{\;����\;}}{\;}$Na2SiO3+CO2����CaCO3+SiO2CaSiO3+CO2����
��2������������Ҫ�ɷ�Ϊ�����ơ���������ȣ�����������������Ʒ�Ӧ���ɹ����ƺ�ˮSiO2+2NaOH=Na2SiO3+H2O����������������ʣ�������������ճ�ᣬ���״�����ʢװNaOH��Һ���Լ�ƿ����Ƥ�������ò�������
�ʴ�Ϊ��SiO2+2NaOH=Na2SiO3+H2O��
��3�����ټ�����������Ź㷺Ӧ�ã�����Ȼ���в���������̬�Ĺ裬��ҵ�����ö��������Ƶôֹ裬�÷�Ӧ�Ļ�ѧ����ʽΪ��SiO2+2C$\frac{\underline{\;����\;}}{\;}$Si+2CO�����ʴ�Ϊ��SiO2+2C$\frac{\underline{\;����\;}}{\;}$Si+2CO����
���� ���⿼��ѧ����ѧ����ʽ����д֪ʶ��ע���������ʵ������ǹؼ����Ѷ��еȣ�

| A�� | 48��O2��O3�Ļ���ﺬ����ԭ����3NA | |
| B�� | 22.4L���飨CH4��������ԭ����Ϊ5NA | |
| C�� | ���ʵ���Ũ��Ϊ0.5 mol/L��MgCl2��Һ�У�����Cl- ����Ϊ NA | |
| D�� | ��20�棬1.01��105Pa�����ڱ�״����ʱ��11.2L����������ԭ����ΪNA |
| A�� | M��ȫ������N��Ϊ4s2��ԭ�Ӻͺ�������Ų�Ϊ1s22s22p63s23p63d64s2 ��ԭ�� | |
| B�� | 2p�ܼ���һ��δ�ɶԵ��ӵĻ�̬ԭ�Ӻ�ԭ�ӵļ۵����Ų�Ϊ2s22p5 | |
| C�� | 3p�ܼ���һ���չ���Ļ�̬ԭ�Ӻͺ�����ӵ��Ų�Ϊ1s22s22p63s23p2 | |
| D�� | �����������Ǻ������������1/5��ԭ�Ӻͼ۵����Ų�Ϊ4s24p1��ԭ�� |
| A�� | ��ԭ�ӵ�������Ϊ222 | B�� | Rn��86��Ԫ�� | ||
| C�� | һ��222 86Rnԭ������136������ | D�� | һ��222 86Rnԭ������136������ |
| A�� | CaCO3+2HCl�TCaCl2+H2O+CO2�� | B�� | BaCl2+Na2SO4�T2NaCl+BaSO4�� | ||
| C�� | 2NaOH+CO2�TNa2CO3+H2O | D�� | Zn+CuSO4�TZnSO4+Cu |
| A�� |  ����ҩƷ | B�� | 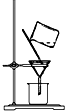 ���� | C�� |  �����Ҵ���ˮ | D�� |  �ռ����� |
| A�� |  ʵ��������ϩ | B�� |  ������Ӧ | ||
| C�� | 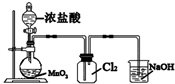 ʵ������ȡCl2 | D�� |  �к��ȵIJⶨ |

�����ķ�Ӧ���£�
CH3CH2CH2CH2OH$��_{H_{2}SO_{4}��}^{Na_{2}Cr_{2}O_{7}}$CH3CH2CH2CHO
��Ӧ��Ͳ��������������£�
| �е�/�� | �ܶȣ�g��cm-3�� | ˮ���ܽ��� | |
| ������ | 117.2 | 0.8109 | �� |
| ����ȩ | 75.5 | 0.8107 | �� |
�ٽ�6.0gNa2Cr2O7����100mL�ձ��У���30mLˮ�ܽ⣬�ٻ�������5mLŨ���ᣬ��������ҺС��ת����B�У�
����A�м���4.0g�������ͼ�����ʯ�����ȣ�������������ʱ����ʼ�μ�B����Һ���μӹ����б��ַ�Ӧ�¶�Ϊ90��95�棬��E���ռ�90�����µ���֣�
�۽�����ﵹ���Һ©���У���ȥˮ�㣬�л������������ռ�75��77����֣�����2.0g��
�ش��������⣺
��1��B�����������ǵ�Һ©����D������������ֱ�������ܣ�
��2����ʯ�������Ƿ�ֹ���У�
��3��������ȩ�ֲ�Ʒ���ڷ�Һ©���з�Һʱ��ˮ���²㣨��ϡ����¡���
��4����Ӧ�¶�Ӧ������90��95�棬��ԭ���DZ�֤����ȩ��ʱ�������ֿɾ��������䱻��һ��������
��5����ʵ���У�����ȩ�IJ���Ϊ51%��