��Ŀ����
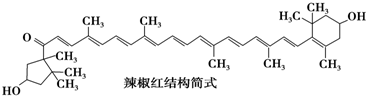
1�� �絼�ʿ����ں����������Һ�ĵ������������絼��Խ������Һ�ĵ�������Խǿ�����õ絼�ʴ����������0.100mol/L��NaOH��Һ�ζ�10.00mL0.100mol/L�����ᣬ�絼��������ͼ��ʾ������˵������ȷ���ǣ�������
�絼�ʿ����ں����������Һ�ĵ������������絼��Խ������Һ�ĵ�������Խǿ�����õ絼�ʴ����������0.100mol/L��NaOH��Һ�ζ�10.00mL0.100mol/L�����ᣬ�絼��������ͼ��ʾ������˵������ȷ���ǣ�������| A�� | d����Һ�У�c��Cl-��=2c��OH-��-2c��H+�� | |
| B�� | �絼�ʴ���������������к͵ζ��յ���ж� | |
| C�� | c��絼����С����Ϊc����Һ������Ϊ������� | |
| D�� | c��Na+����c��Cl-���Ե���������Ӱ���c��H+����c��OH-���Ե���������Ӱ��С |
���� A���κε������Һ�ж����ڵ���غ㡢�����غ㣬���ݵ���غ㼰�����غ��жϣ�
B��HCl��NaOH��Ӧ����ʽΪHCl+NaOH=NaCl+H2O������ͼ��֪����0-10mL֮�䣬���ŷ�Ӧ�Ľ��У���Һ��c��H+��Ũ����С����Һ�ĵ絼�����ͣ�������Һ�������15mLʱ����Һ������������Ũ����������Һ�ĵ絼��������ͼ֪����ǡ���к�ʱ�絼����С��
C����Һ�絼��������Ũ�ȳ����ȣ�c��絼�ʵ�����Ϊ����Ũ��С��
D��HCl��NaOH��Ӧ����ʽΪHCl+NaOH=NaCl+H2O������ͼ��֪����0-10mL֮�䣬���ŷ�Ӧ�Ľ��У���Һ��c��H+��Ũ����С���¶Ȳ������ӻ��������䣬����c��OH-������c��H+����c��OH-������С����Һ�ĵ絼�����ͣ�������Һ�������15mLʱ����Һ��c��OH-����������Һ�ĵ絼������0-15mL�����У�c��Cl-����c��Na+��ʼ�մ���c��H+����c��OH-����
��� �⣺A���κε������Һ�ж����ڵ���غ㡢�����غ㣬���ݵ���غ��c��Na+��+c��H+��=c��Cl-��+c��OH-����d��NaOH��HCl�����֮��Ϊ3��2���������ʵ���Ũ����ȣ����������غ��2c��Na+��=3c��Cl-�������Ե�c��Cl-��=2c��OH-��-2c��H+����
��A��ȷ��
B��HCl��NaOH��Ӧ����ʽΪHCl+NaOH=NaCl+H2O������ͼ��֪����0-10mL֮�䣬���ŷ�Ӧ�Ľ��У���Һ��c��H+��Ũ����С����Һ�ĵ絼�����ͣ�������Һ�������15mLʱ����Һ������������Ũ����������Һ�ĵ絼��������ͼ֪��ǡ���к�ʱ�絼����С�����Կ��Ե絼�ʴ���������������к͵ζ��յ���жϣ���B��ȷ��
C���絼��������Ũ�ȳ����ȣ�c��絼�ʵ�����Ϊ����Ũ��С��c���Ƕ���ǡ����ȫ��Ӧ����NaCl��NaCl��ǿ����ʣ���C����
D��HCl��NaOH��Ӧ����ʽΪHCl+NaOH=NaCl+H2O������ͼ��֪����0-10mL֮�䣬���ŷ�Ӧ�Ľ��У���Һ��c��H+��Ũ����С���¶Ȳ������ӻ��������䣬����c��OH-������c��H+����c��OH-������С����Һ�ĵ絼�����ͣ�������Һ�������15mLʱ����Һ��c��OH-����������Һ�ĵ絼������0-15mL�����У�c��Cl-����c��Na+��ʼ�մ���c��H+����c��OH-�����ɴ˿ɼ�c��Na+����c��Cl-���Ե���������Ӱ���c��H+����c��OH-���Ե���������Ӱ��С����D��ȷ��
��ѡC��
���� �����Ե������Һ�ĵ�����Ϊ���忼���������Һ�����жϣ���ȷ��Ӧʵ�ʡ��絼�ʵ�Ӱ�����ؼ������Һ�������ǽⱾ��ؼ��������ͼ�������߱仯��ȡ��Ϣ���ӹ���Ϣ���Ӷ�������Ϣ���⣬�״�ѡ����A��

 �ŵ������ϵ�д�
�ŵ������ϵ�д� 53������ϵ�д�
53������ϵ�д�| A�� | 1���� | B�� | �Ҵ� | C�� | 1���� | D�� | 1�촼 |
| ����� | �Լ� | ���뷽�� | |
| A | �屽���壩 | �������� | ��Һ |
| B | ���飨��ϩ�� | ���� | ���������� |
| C | �������������ᣩ | ����̼���� | ��Һ |
| D | �Ҵ���ˮ�� | ��ʯ�� | ���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
��Fe2+ת��ΪFe3+����O2����ԭ���۲���H2��Fe��OH��3ʧˮ�γ�Fe2O3•nH2O��������̼��������
| A�� | ֻ�Тٺ͢� | B�� | ֻ�Тڢۢ� | C�� | �٢ڢۢ� | D�� | �٢ڢۢܢ� |
| A�� | ���ܷ���������Ӧ | B�� | ���ڷ����廯���� | ||
| C�� | �ܷ�����ȥ��Ӧ | D�� | �����к���11��̼̼˫�� |

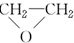 ���������飩��
���������飩��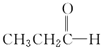 $\stackrel{NaBH_{4}}{��}$CH3CH2CH2OH
$\stackrel{NaBH_{4}}{��}$CH3CH2CH2OH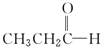 $��_{HCl}^{Zn��Hg��}$CH3CH2CH3
$��_{HCl}^{Zn��Hg��}$CH3CH2CH3