��Ŀ����
����Ŀ���������[aFe2(SO4)3��b(NH4)2SO4��cH2O]�㷺���ڳ�����������ˮ����ҵѭ��ˮ�ľ����ȡ�ij����������������(�����������)�������Ϊԭ�ϣ������ͼ����������ȡ������李�
��ش��������⣺
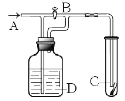
(1)����������Һ�������ữ����ҪĿ����________��
(2)�������������ʺϵ�������B��________��
a��NaClO b��H2O2 c��KMnO4 d��K2Cr2O7
(3)������������_____��
(4)���������У�����֮��ͼ�������֮ǰ�����������______(�ѧʽ)������Fe2���Ƿ���ȫ���������ķ���Ϊ_______________��
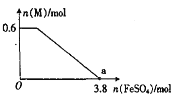
(5)��ȡ14.00 g���ò�Ʒ����������ˮ���Ƴ�100 mL��Һ���ֳ����ȷݣ�������һ����Һ�м�������NaOH��Һ�����ˡ�ϴ�ӵõ�2.14 g����������һ����Һ�м��뺬0.05 mol Ba(NO3)2����Һ��ǡ����ȫ��Ӧ�����������淋Ļ�ѧʽΪ____��
���𰸡�������Һ��SO42-��Ũ�ȣ���Ca2��ת��Ϊ������ͬʱ����Fe2��ˮ�� b ��ȴ�ᾧ H2SO4 ����K3[Fe(CN)6]��Һ����������ɫ��������˵����������δȫ�������� Fe2(SO4)3��2(NH4)2SO4��2H2O
��������
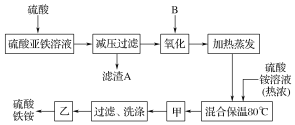
ԭ���м��������ữ������������Ƴ�������ѹ���˺�������������������������������������泥���80���·�Ӧ������������泥�������Ũ������ȴ�ᾧ������ϴ�ӣ��������ɹ�ɵ�������������泥�
��1��������������������ˮ�⣬�����������ˮ�⣬��������ӿ��Խ������ӳ�����
��2�������������������������ӣ���Ӧ�������������ʣ�
��3��������茶�����Ũ������ȴ�ᾧ������ϴ�ӣ�
��4�����������У�����֮��ͼ�������֮ǰ����ֹ������ˮ�⣬�����軯����Һ���Լ����������ӵĴ��ڣ�
��5������Ԫ���غ��Ϸ�����ת�������㡣
��1���������ᣬ��������Һ��SO42Ũ�ȣ���Ca2+ת��Ϊ����������CaSO4��ͬʱ����Fe2+ˮ�⣻
�ʴ�Ϊ��������Һ��SO42-��Ũ�ȣ���Ca2��ת��Ϊ������ͬʱ����Fe2��ˮ�⣻
��2��Ϊ�������������ʣ�Ӧ�����������Ϊ����������ԭ������ˮ��
�ʴ�Ϊ��b��
��3����80���·�Ӧ������������泥�������Ũ������ȴ�ᾧ������ϴ�ӣ����Բ�����Ϊ��ȴ�ᾧ��
�ʴ�Ϊ����ȴ�ᾧ��
��4�����������У�����֮��ͼ�������֮ǰ��Ϊ��ֹ������ˮ�⣬Ҫ���������������Fe2+�Ƿ���ȫ���������ķ���������Һ�м���K3[Fe(CN)6]��Һ����������ɫ��������˵�������������ӣ���˵����������δȫ����������
�ʴ�Ϊ��H2SO4������K3[Fe(CN)6]��Һ����������ɫ��������˵����������δȫ����������
��5����ȡ14.00g��Ʒ����������ˮ���ó�100mL��Һ���ֳ����ȷݣ�������һ���м�������NaOH��Һ������ϴ�ӵõ�2.14g������ӦΪFe(OH)3��n[Fe(OH)3]=![]() ������һ����Һ�м���0.05molBa(NO3)2��Һ��ǡ����ȫ��Ӧ����n(SO42)=0.05mol������14.00g��Ʒ�к���Fe2(SO4)3Ϊ0.02mol��n(SO42)Ϊ0.1mol����(NH4)2SO4Ϊ0.1mol0.02mol��3=0.04mol����m(H2O)=14.00g0.02mol��400g/mol0.04mol��132g/mol=0.72g��n(H2O)=
������һ����Һ�м���0.05molBa(NO3)2��Һ��ǡ����ȫ��Ӧ����n(SO42)=0.05mol������14.00g��Ʒ�к���Fe2(SO4)3Ϊ0.02mol��n(SO42)Ϊ0.1mol����(NH4)2SO4Ϊ0.1mol0.02mol��3=0.04mol����m(H2O)=14.00g0.02mol��400g/mol0.04mol��132g/mol=0.72g��n(H2O)=![]() ��n[Fe2(SO4)3]:n[(NH4)2SO4]:n(H2O)=0.02:0.04:0.04=1:2:2�����Ի�ѧʽΪFe2(SO4)3��2(NH4)2SO4��2H2O��
��n[Fe2(SO4)3]:n[(NH4)2SO4]:n(H2O)=0.02:0.04:0.04=1:2:2�����Ի�ѧʽΪFe2(SO4)3��2(NH4)2SO4��2H2O��
�ʴ�Ϊ��Fe2(SO4)3��2(NH4)2SO4��2H2O��

 ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д�����Ŀ����þ����һ�ֹ�ҵ���ϣ���Ҫ�ɷ���MgO(ռ40%)������CaO��MnO��Fe2O3��FeO��Al2O3�����ʣ��Դ�Ϊԭ����ȡ������þ������ӡȾ����ֽ��ҽҩ�ȹ�ҵ������þ������ȡMgSO4��7H2O���������£�
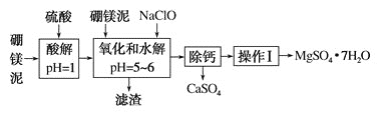
��������ش��������⣺
��1��ʵ��������1 mol��L��1������900 mL������ʱ���õIJ������������ձ�������������Ͳ�⣬����________________��
��2�������NaClO�ɽ�Mn2������ΪMnO2 ����Ӧ���������������Ӧ�����ӷ���ʽΪ��________________������һ������Ҳ�ᱻNaClO��������Ӧ�����ӷ���ʽΪ_______________________��
��3������������ǰ���������Һ��Fe3���Ƿ������������鷽����___________________��
��4����֪MgSO4��CaSO4���ܽ�����±���
�¶�/�� | 40 | 50 | 60 | 70 |
S(MgSO4)/g | 30.9 | 33.4 | 35.6 | 36.9 |
S(CaSO4)/g | 0.210 | 0.207 | 0.201 | 0.193 |
���������ǽ�MgSO4��CaSO4�Ļ����Һ�е�CaSO4��ȥ�������ϱ����ݣ���ȷ����������Ϊ�����ᾧ��_______________________����������ƣ���