��Ŀ����
����Ŀ����ѧʵ���ǻ�ѧѧϰ����Ҫ���ݡ����������յ�֪ʶ������������ݡ�
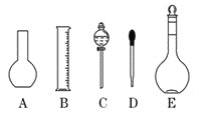
(1)ʵ������Ҫ0.1 mol/L NaOH��Һ480 mL�� ������Һ����������ش��������⣺
����ͼ��ʾ��������������Һ�϶�����Ҫ����________(�����)��
���ɼ����֪��Ӧѡ��________mL������ƿ����������ƽ��ȡ________g NaOH��
��������NaOH��Һʵ���У�������������ȷ��������ʱ���ӿ̶��ߣ� ����������ҺŨ��________0.1 mol/L (����������������������С��������ͬ)���ܽ�NaOH�����Һ����ȴ�����º���ת��������ƿ�С�������ת�ƻ�ʹ��������ҺŨ��________0.1 mol/L��
(2)ѡ������ʵ�鷽���������ʣ������뷽����������ں����ϡ�
A.��ȡ��Һ B.���� C.�����ᾧ D.���� E.���� F.��Һ
�ٳ�ȥϴ��ˮ��IJ�Ҷ�Ӻ�����________��
�ڴ�ʳ��ˮ����ʳ�ι���________��
�۴ӵ��ɳ�ӻ�����л�õ�________��
�ܴ���ˮ�л����________��
�ݷ���CCl4 �е�Ϊ76.75��ͼױ��е�Ϊ110.6��Ļ����________��
���𰸡�AC 500mL 2.0g С�� ���� D C B A E
��������
(1)��������һ�����ʵ���Ũ�ȵ���Һ��������ȷ��ʹ�õ�������
�ڸ���ѡ������ƿ�Ĺ������n=c��V�������ʵ����ʵ������ٸ���m=n��M��������������ʵ�������
�۸���c=![]() ����ʵ����
����ʵ����
(2)�ٸ��ݲ�Ҷ��������������ˮ�Ĺ��������
�ڸ���NaCl��ˮ���ܽ�����¶ȵ�Ӱ��仯���������
�۸��ݵⵥ�ʼ��Ȼ�ֱ���ɹ�̬��Ϊ��̬������
�ܸ����嵥�����л����������ܽ����ˮ���ܽ�ȱȽ�С��ˮ���л��ﻥ�����ܷ�����
�ݸ��ݻ��ܵ�����Һ�����ʷе�IJ�ͬ���롣
(1)������0.1mol/L��NaOH��Һ480mL������û�й����480mL������ƿ����˸���ѡ�������Ĺ���Ҫʹ��500mL������ƿ��������Һ�IJ���Ϊ���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ�������ƹ��������������Ϊ������ƽ��ҩ�ס��ձ�������������Ͳ��500mL����ƿ����ͷ�ιܣ���˲����õ�������Ϊ��ƿ(A)�ͷ�Һ©��(C)��
��ѡ������ƿ�Ĺ����500mL��������Һ��Ũ����0.1mol/L������n=cV��֪��NaOH�����ʵ���n(NaOH)=cV=0.1mol/L��0.5L=0.05mol����m=nM��֪�����NaOH������m(NaOH)=0.05mol��40g/mol=2.0g��
��������ʱ���ӿ̶��ߣ���������Һ�����ƫ��Vƫ���ݹ�ʽc=![]() ��֪��������Һ�����ʵ���Ũ��ƫС��
��֪��������Һ�����ʵ���Ũ��ƫС��
������ת����Һ������Һ�����������������������Һ�����ƫС����VƫС�����ݹ�ʽc=![]() ��֪��������Һ�����ʵ���Ũ��ƫ��
��֪��������Һ�����ʵ���Ũ��ƫ��
(2)�ٲ�Ҷ�Ӻ�����������ˮ����˷����Ҷ�Ӻ�������Ӧ���ù��˲���������ѡ����D��
�ڴ���Һ�л�����ʹ��壬Ӧ���ýᾧ������˴�ʳ��ˮ�л��ʳ�ι��壬Ӧ���������ᾧ������ѡ����C��
�۵���������������ɳ�Ӳ�����������˷�����ɳ�ӻ����ɲ���������õⵥ�ʣ��ʺ���ѡ����B��
�������л��ܼ��е��ܽ�Ƚϴ���ˮ���ܽ��С���л�����ˮ�������ܣ���˿ɲ�������ȡȻ����з�Һ�ķ�������ˮ�л���嵥�ʣ��ʺ���ѡ����A��
��CCl4�ͼױ�Ϊ���ֻ��ܵ�Һ�壬�Ҷ��ߵķе����ϴɲ�������ķ������룬�ʺ���ѡ����E��