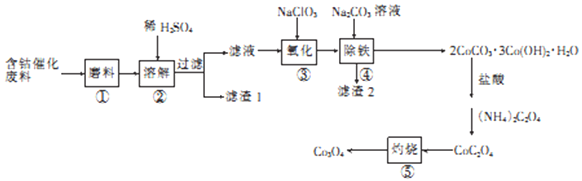
��Ŀ����
����Ŀ��Ni2O3��Ҫ�����մɡ��´ɺͲ�������ɫ���ϣ�Ҳ���������۵����죬����һ��������������(��֪��ԭ�ԣ�Fe2+>Ni2+)��
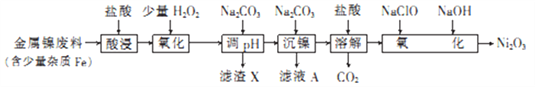
�ش��������⣺
(1) Ni2O3��Ni �Ļ��ϼ�Ϊ________________��
(2)Ϊ����߽��������Ͻ������ʣ��ڡ������ʱ�ɲ�ȡ�Ĵ�ʩ��_______________________(д����)��
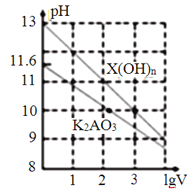
(3)����H2O2��Һ��Ϊ����KMnO4��Һ(�ڸ�ʵ�������£�Ni2+���ܱ�KMnO4����)���������������е����ӷ���ʽΪ_______________________������Na2CO3��Һ��pH�������ġ�����X����_____________��
(4)����ҺA�пɻ������õ���Ҫ������___________________������NaClO��Һ��NaOH��Һ��������������Ni2O3�����ӷ���ʽΪ_________________________��
(5)��ҵ������Ϊ���������0.05��0.1mol��L-1NiCl2��Һ��һ����NH4Cl��ɵĻ����Һ���ɵõ��ߴ��ȡ����εij�ϸ���ۡ�����������һ��ʱ��NH4Cl ��Ũ�ȶ���������Ч�ʼ����ijɷ��ʵ�Ӱ������ͼ��ʾ��
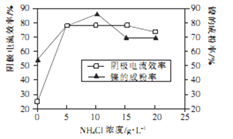
��NH4ClŨ����ÿ���Ϊ____________g��L-1��
�ڵ�NH4ClŨ�ȴ���15g��L-1ʱ���������������ɣ�������������Ч�ʽ��ͣ���Ӧ�ĵ缫��Ӧʽ
Ϊ______________________��
���𰸡� +3 �ʵ������¶ȣ����������Ũ�ȣ�������������гɷ�ĩ�� 5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O Fe(OH)3 NaCl 2Ni2++ClO-+4OH-=Ni2O3��+Cl-+2H2O 10 2H++2e-=H2��(��2NH4++2H2O+2e-=2NH3��H2O+H2��
�����������⿼�黯ѧ�������̣�Ni2O3��O�ԣ�2�ۣ���Ni�ļ�̬Ϊ��3�ۣ���2����߽����ʵĴ�ʩ���ʵ������¶ȣ����������Ũ�ȣ�������������гɷ�ĩ�ȣ���3���������ķ����к���Fe���������ᷴӦ����FeCl2��������ؾ���ǿ�����ԣ��ܰ�Fe2��������Fe3������������ԭ��Mn2�������ӷ�Ӧ����ʽΪ��5Fe2��+MnO4��+8H��=5Fe3��+Mn2��+4H2O������Na2CO3��Ŀ���ǵ���pH����Fe3����������������ʽ�����������������XΪFe(OH)3����4�������ܽ���̣�����CO2����˳������������ӷ�Ӧ��Ni2����CO32��=NiCO3������ҺA�ijɷ���NaCl������Na2CO3������NaClO��Һ������ClO����ǿ�����ԣ���Ni2��������Ni3����������ӷ�Ӧ����ʽΪ2Ni2��+ClO��+4OH��=Ni2O3��+Cl��+2H2O����5���ٸ���ͼ��NH4Cl��Ũ��Ϊ10g��L��1ʱ����������Ч���Լ����ijɷ��ʴﵽ��ߣ��ڸ������⣬���ﵽ15g��L��1ʱ�������������������������ӦʽΪ2H����2e��=H2������2NH4��+2H2O+2e��=2NH3��H2O+H2����

 ��Ӣ���㿨ϵ�д�
��Ӣ���㿨ϵ�д� Ӧ����㲦ϵ�д�
Ӧ����㲦ϵ�д� ״Ԫ����ϵ�д�
״Ԫ����ϵ�д�