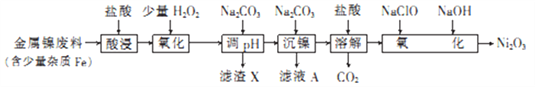
��Ŀ����
����Ŀ����.����������������BF3����HCN����NH2- ����д���пհ�(�����)��
��1�����ڼ��Լ��ķǼ��Է�����__________��
��2������ԭ�ӹ��Ϊsp3�ӻ�����________��
��3��ֻ��������������__________�� ��������������__________��
��4���ռ乹�ͳʡ�V���ε���__________��
��.�������γɶ������ӣ���N3����NH2����N3����NH4+��N2H5+��N2H62+�ȣ���֪N2H5+��N2H62+�������Է��ӽ�������γɵģ�������NH4+������������� NH4+�����ʡ�
��1��N2H62+�ڼ�����Һ�з�Ӧ�����ӷ���ʽ��_______________��
��2��NH2���ĵ���ʽΪ__________________________��
��3��д��������N3���ȵ����������ѧʽ___________��
���𰸡� �� �� �٢� �� �� N2H62++2OH����N2H4+2H2O ![]() N2O CO2 CNO���Ⱥ�����
N2O CO2 CNO���Ⱥ�����
��������I.��BF3��Bԭ����Fԭ���Լ��Լ����ϣ�Bԭ�ӵŵ��Ӷ���Ϊ�� ![]() =0���Ҽ���Ϊ3������ԭ�ӵ��ӻ�����Ϊsp2�ӻ����ռ乹��Ϊ�������Σ����ڷǼ��Է��ӣ���HCN��Cԭ����Hԭ���Ե������ϣ�Cԭ�Ӻ�Nԭ����������������Ϊ���Լ�������Cԭ���µ��Ӷԣ������к���2���Ҽ���2������������Cԭ��Ϊsp�ӻ�����һ��ֱ���εļ��Է�������NH2��Nԭ�Ӻ�Hԭ���Լ��Լ����ϣ�Nԭ�ӵŵ��Ӷ���Ϊ��
=0���Ҽ���Ϊ3������ԭ�ӵ��ӻ�����Ϊsp2�ӻ����ռ乹��Ϊ�������Σ����ڷǼ��Է��ӣ���HCN��Cԭ����Hԭ���Ե������ϣ�Cԭ�Ӻ�Nԭ����������������Ϊ���Լ�������Cԭ���µ��Ӷԣ������к���2���Ҽ���2������������Cԭ��Ϊsp�ӻ�����һ��ֱ���εļ��Է�������NH2��Nԭ�Ӻ�Hԭ���Լ��Լ����ϣ�Nԭ�ӵŵ��Ӷ���Ϊ�� ![]() =2���Ҽ���Ϊ2������ԭ�ӵ��ӻ�����Ϊsp3�ӻ����ռ乹��ΪV����
=2���Ҽ���Ϊ2������ԭ�ӵ��ӻ�����Ϊsp3�ӻ����ռ乹��ΪV����
(1). ������������֪�����ڼ��Լ��ķǼ��Է�����BF3���ʴ�Ϊ������
(2). ����ԭ�ӹ��Ϊsp3�ӻ�����NH2���ʴ�Ϊ������
(3). ֻ���ڦҼ�������BF3��NH2�� ���ڦм�������HCN���ʴ�Ϊ���٢�������
(4). �ռ乹�ͳʡ�V���ε���NH2���ʴ�Ϊ������
II. (1). N2H62+�������Է���N2H4���2�������γɵģ���N2H62+�൱�ڶ�Ԫ�ᣬ��������OH��Ӧʱ�����ʵ���֮��Ϊ1:2���ڼ�����Һ�з�Ӧ�����ӷ���ʽΪ��N2H62++2OH����N2H4+2H2O���ʴ�Ϊ��N2H62++2OH����N2H4+2H2O��
(2). NH2�еĵ�ԭ�������5�������е�����������2����ԭ���γɹ��ۼ������ٵõ�1�����Ӵﵽ����ȶ��ṹ���������ʽΪ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(3). N3���к���3��ԭ�ӡ��۵�����Ϊ16�����ݵȵ�����ԭ������N3��Ϊ�ȵ������������N2O��CO2��CNO-�ȣ��ʴ�Ϊ��N2O��CO2��CNO-�ȡ�