��Ŀ����
����Ŀ����ͼ��ʾ��һ�ܱ���������Ħ�����ɻ�����������A��B�ֳɼס������ҡ���״���£������ҳ���8.4g N2�����ҳ���8.8g H2��O2�Ļ�����塣

��1�������е�ԭ�Ӹ���ԼΪ ______________����H2��O2��������Ϊ_____________��
��2��ά���¶Ȳ��䣬������A�̶��ڡ�3������������B�̶��ڡ�5��������ʱ�ס��������������ѹǿ��P���ף���P���ң�=______________��
��3���õ���������е�����ʹ���ַ�Ӧ����Ӧ��ָ�����״�������������Ҵ�ʱ�������Ϊ__________��
���𰸡�4.816��1023��0.8��6.02��1023����0.8NA���÷֣�1:328:92:3
��������
��1��8.4g���������ʵ�����8.4g��28g/mol��0.3mol���ס�������ѹǿ���¶���ͬ����������ʵ���֮�ȵ������������֮�ȣ���������������ʵ���Ϊ0.3mol��4/3��0.4mol��������������������2��ԭ����ɵĵ��ʣ�������е�ԭ�ӵ����ʵ�����0.8mol������ԭ�Ӹ���ԼΪ0.8��6.02��1023�������������������ʵ����ֱ���xmol��ymol����x+y��0.4��2x+32y��8.8�����x��2/15��y��4/15������H2��O2��������Ϊ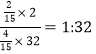 ��
��
��2��ά���¶Ȳ��䣬������A�̶��ڡ�3�����������ѹǿ֮�������ʵ���֮�ȿ�֪��ʱ����ѹǿ��ԭ����4/3��������B�̶��ڡ�5��������ʱ����ѹǿ��ԭ����3/2������Ϊԭ�������е�ѹǿ��ȣ����ʱ�ס��������������ѹǿ��P���ף���P���ң�=4/3:3/2=8:9��
��3����2H2+O2![]() 2H2O�Լ����Ϸ�����֪��Ӧ��������ʣ�࣬ʣ������Ϊ4/15mol-1/15mol=0.2mol���ָ�ԭ�¶Ⱥ�����������ѹǿ��ȣ����֮�ȵ��������ʵ���֮�ȣ���ס������ҵ����֮��Ϊ0.2mol��0.3mol=2:3��
2H2O�Լ����Ϸ�����֪��Ӧ��������ʣ�࣬ʣ������Ϊ4/15mol-1/15mol=0.2mol���ָ�ԭ�¶Ⱥ�����������ѹǿ��ȣ����֮�ȵ��������ʵ���֮�ȣ���ס������ҵ����֮��Ϊ0.2mol��0.3mol=2:3��

 �����ܿ����ϵ�д�
�����ܿ����ϵ�д�