��Ŀ����
8�����ʵ����ͺ���Ԫ�صĻ��ϼ����о��������ʵ�������Ҫ�ӽǣ����仯�������̬�仯Ϊ����Ķ�άת����ϵ��ͼ1��ʾ��
���������գ�
��1��ͼ��X�ĵ���ʽΪ
 ����ˮ��Һ�ڿ����з����ױ���ǣ�д����Ӧ�Ļ�ѧ����ʽ2H2S+O2��2S��+2H2O���ñ仯˵��S�ķǽ����Ա�O�����ǿ��������������ԭ�ӽṹ�ĽǶȽ���ԭ����ԭ�Ӱ뾶������ԭ�ӣ���ԭ�ӵĵ�������С����ԭ�ӣ�ͨ��H2O���ȶ��Դ���H2S��SO2������+4�ۣ�����-2�ۣ�����������Ҳ�����ж�����������Ԫ�صķǽ�����ǿ����
����ˮ��Һ�ڿ����з����ױ���ǣ�д����Ӧ�Ļ�ѧ����ʽ2H2S+O2��2S��+2H2O���ñ仯˵��S�ķǽ����Ա�O�����ǿ��������������ԭ�ӽṹ�ĽǶȽ���ԭ����ԭ�Ӱ뾶������ԭ�ӣ���ԭ�ӵĵ�������С����ԭ�ӣ�ͨ��H2O���ȶ��Դ���H2S��SO2������+4�ۣ�����-2�ۣ�����������Ҳ�����ж�����������Ԫ�صķǽ�����ǿ������2��������������Na2S2O3�Ʊ�����������ԭ��Ӧ�ĽǶȣ��������п��ܵ���bd��ѡ���ţ���
a��Na2S+S b��Z+S c��Na2SO3+Y d��NaHS+NaHSO3
��3����֪��Ӧ��Na2S2O3+H2SO4��Na2SO4+S��+SO2+H2O���о��䷴Ӧ����ʱ�����з�����������b��ѡ���ţ���
a���ⶨһ��ʱ��������SO2��������ó��÷�Ӧ������
b���о�Ũ�ȡ��¶ȵ����ضԸ÷�Ӧ���ʵ�Ӱ�죬�ȽϷ�Ӧ���ֻ��ǵ�ʱ��
c����Na2S2O3����ֱ���Ũ��ϡ���ᷴӦ���о�Ũ�ȶԸ÷�Ӧ���ʵ�Ӱ��
��4��������CO��SO2���̵�������Fe2O3����������CO��SO2��380��ʱת��ΪS��һ�������壮��֪��������۵㣺112.8�桢�е㣺444.6�棻�ڷ�Ӧÿ�õ�1mol�ų�270kJ��������д���������̵�����Ӧ���Ȼ�ѧ����ʽ2CO��g��+SO2��g��$\frac{\underline{\;\;\;F_{2}O_{3}\;\;\;}}{380��}$S��l��+2CO2 ��g����H=-270kJ/mol��
��5������������ͬ��������ͬʱ��������Ӧ��SO2��ת�����淴Ӧ�¶ȵı仯��ͼ2�������Ǵ����۸����أ�������ѡFe2O3����������Ҫԭ����Fe2O3������������Խϵ��¶��£���λʱ���ڻ�ýϸߵ�SO2ת���ʣ��ܺ�С��
���� ��1��XΪH2S��S�����6�����ӣ��ܹ���2��Hԭ���γɹ��ۼ���H2S�ڿ����б��������Ϊ����������ΪS��ͬ����Ԫ��������������ͬ��ԭ�Ӱ뾶���϶��������õ�������������ʧ������������ǿ��
��2��Na2S2O3��SΪ+2�ۣ���������ԭ�ĽǶȷ�������Ӧ����SԪ�ػ��ϼ۱���ֱ����2��С��2��
��3�������������ϡ���ᷴӦ�����˵�������Һ����ǣ���Ӧ����Խ�죬���ֻ���ʱ��Խ�̣�
��4����Ӧÿ�õ�1mol�ų�270kJ���������Դ���д�Ȼ�ѧ����ʽ��
��5����ͼ��֪����ͬ������������������ʱ�����������ת�������
��� �⣺��1��XΪH2S��S�����6�����ӣ��ܹ���2��Hԭ���γɹ��ۼ��������ʽΪ��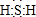 ��H2S�ڿ����б��������Ϊ����������ΪS����Ӧ�Ļ�ѧ����ʽΪ2H2S+O2��2S��+2H2O������S�ǽ����Ա�O����ͬ����Ԫ��������������ͬ��ԭ�Ӱ뾶���϶��������õ�������������ʧ������������ǿ������ԭ�Ӱ뾶������ԭ�ӣ���ԭ�ӵĵ�������С����ԭ�ӿɽ��ͣ�ʵ����H2O���ȶ��Դ���H2S��SO2������+4�ۣ�����-2�ۣ�
��H2S�ڿ����б��������Ϊ����������ΪS����Ӧ�Ļ�ѧ����ʽΪ2H2S+O2��2S��+2H2O������S�ǽ����Ա�O����ͬ����Ԫ��������������ͬ��ԭ�Ӱ뾶���϶��������õ�������������ʧ������������ǿ������ԭ�Ӱ뾶������ԭ�ӣ���ԭ�ӵĵ�������С����ԭ�ӿɽ��ͣ�ʵ����H2O���ȶ��Դ���H2S��SO2������+4�ۣ�����-2�ۣ�
�ʴ�Ϊ��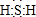 ��������ԭ�Ӱ뾶������ԭ�ӣ���ԭ�ӵĵ�������С����ԭ�ӣ�H2O���ȶ��Դ���H2S��SO2������+4�ۣ�����-2�ۣ�
��������ԭ�Ӱ뾶������ԭ�ӣ���ԭ�ӵĵ�������С����ԭ�ӣ�H2O���ȶ��Դ���H2S��SO2������+4�ۣ�����-2�ۣ�
��2��Na2S2O3��SΪ+2�ۣ���������ԭ�ĽǶȷ�������Ӧ����SԪ�ػ��ϼ۱���ֱ����2��С��2��a��S���ϼ۶�С��2��c��S�Ļ��ϼ۶�����2��bd�������⣬
�ʴ�Ϊ��bd��
��3�����������������ϡ���ᷴӦ�����˵�������Һ����ǣ������жϷ�Ӧ���ʿ�������Ӧ����Խ�죬���ֻ���ʱ��Խ�̣��ʴ�Ϊ��b��
��4����Ӧÿ�õ�1mol�ų�270kJ���������Ȼ�ѧ����ʽΪ2CO��g��+SO2��g��$\frac{\underline{\;\;\;F_{2}O_{3}\;\;\;}}{380��}$S��l��+2CO2 ��g����H=-270kJ/mol��
�ʴ�Ϊ��2CO��g��+SO2��g��$\frac{\underline{\;\;\;F_{2}O_{3}\;\;\;}}{380��}$S��l��+2CO2 ��g����H=-270kJ/mol��
��5����ͼ��֪��������ѡFe2O3����������Ҫԭ����Fe2O3������������Խϵ��¶��£���λʱ���ڻ�ýϸߵ�SO2ת���ʣ��ܺ�С��
�ʴ�Ϊ��Fe2O3������������Խϵ��¶��£���λʱ���ڻ�ýϸߵ�SO2ת���ʣ��ܺ�С��
���� ���⿼����ۺϣ��漰�ǽ����ԱȽϡ�������ԭ��Ӧ����Ӧ���ʼ��Ȼ�ѧ����ʽ�ȣ����ط�Ӧԭ���и�Ƶ����Ŀ��飬�ۺ��Խ�ǿ����Ŀ�ѶȲ���

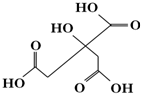
| A�� | X����ʽΪC6H6O7 | |
| B�� | 1 mol����X���Ժ�3 mol���������ӳ� | |
| C�� | X���Ӳ����Է�����ȥ��Ӧ | |
| D�� | ������X�ֱ�������ʵ�����NaHCO3��Na2CO3��Ӧ�õ����������ʵ�����ͬ |
 �ش����Ϊ��֤�ʻ�ʢ�����Դ��������ʻ�ʩ����S=�տ����Ƽ���S-�տ��صķ��ӽṹ��ͼ��ʾ�����й��ڸ����ʵ�˵����ȷ���ǣ�������
�ش����Ϊ��֤�ʻ�ʢ�����Դ��������ʻ�ʩ����S=�տ����Ƽ���S-�տ��صķ��ӽṹ��ͼ��ʾ�����й��ڸ����ʵ�˵����ȷ���ǣ�������| A�� | ���л���ķ���ʽΪC15H22O4 | |
| B�� | 1mol���л���������Na��Ӧ����1molH2 | |
| C�� | ���л����ܷ���ȡ�����ӳɺ�ˮ�ⷴӦ | |
| D�� | 1mol���л����������巴Ӧ�������4molBr2 |
| A�� | 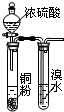 ��֤Ũ�������ǿ������ | B�� |  ��ȡ�����NH3 | ||
| C�� |  ����ռ������ն���SO2 | D�� | 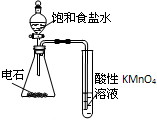 ��֤��Ȳ�Ļ�ԭ�� |
| A�� | �۱�ϩ�����ڣ�-CH2-CH2-CH2- | B�� | ������̼���ӵı���ģ�ͣ�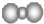 | ||
| C�� | 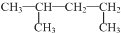 �����ƣ�1��3-�������� �����ƣ�1��3-�������� | D�� | �����ӵĽṹʾ��ͼ�� |
| ��ѧʽ | NH3•H2O | CH3COOH | HCN | H2CO3 |
| Ki��25�棩 | 1.8��l0-5 | 1.8��l0-5 | 4.9��l0-10 | Ki1=4.3��l0-7 Ki2=5.6��l0-11 |
| A�� | �����ʵ���Ũ�ȵ�NaHCO3��NaCN��Һ��ǰ����Һ��ˮ�ĵ���̶ȴ� | |
| B�� | 0.1 mol/L CH3COONa ��Һ�Լ��ԣ�0.1 mol/L CH3COONH4 ��Һ������ | |
| C�� | CN-+H2O+CO2��HCN+HCO3- | |
| D�� | �к͵��������pH��CH3COOH��HCN����NaOH����ǰ��С�ں��� |
| A�� | ���ʹ軯ѧ�����ȶ�������Ȼ�粻��������̬�Ĺ� | |
| B�� | ������ˮ��Һ�ܵ��磬˵�������ǵ���� | |
| C�� | SO2ʹ��ˮ��Ʒ����Һ��ɫ��������SO2��Ư���� | |
| D�� | þ������ͭ�Ƚ���һ������Ȼ�ԭ��ұ�� |
