��Ŀ����
����Ŀ����������ƣ�Na2S2O3��5H2O�����������մ��ֳ�Ϊ������������������ˮ���������Ҵ������ȡ�������ֽ⡣ijʵ����ģ�ҵ���ȡ��������ƣ��䷴Ӧװ�ü������Լ�����ͼ��
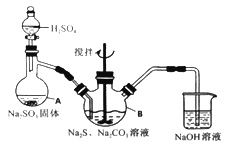
ʵ������������Ϊ��
�ٿ�����Һ©����ʹ�����������£��ʵ����ڷ�Һ�ĵ��٣�ʹ��Ӧ������SO2����Ͼ��ȵ�ͨ��Na2S��Na2CO3�Ļ����Һ�У�ͬʱ�����綯������������ˮԡ���ȣ��С�
��ֱ�������Ļ��Dz�����ʧ����������Һ��pH�ӽ�7ʱ��ֹͣͨ��SO2���塣
����
��1�������A������_______��
��2��Ϊ�˱�֤��������ƵIJ�����ʵ���в�������ҺpH <7���������ӷ���ʽ����ԭ��_________��
��3��д��������ƿB����ȡNa2S2O3����Ӧ���ܻ�ѧ��Ӧ����ʽ________��
��4����������������Һ�л�ýϸ߲���Na2S2O3��5H2O�IJ���Ϊ
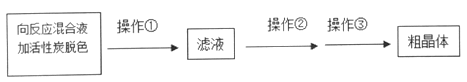
Ϊ���ٲ�Ʒ����ʧ��������Ϊ���ȹ��ˣ�����������ԭ����______����������______���������dz��ˡ�ϴ�ӡ����
��5���ⶨ��Ʒ����
ȡ6.00g��Ʒ�����Ƴ�100mL��Һ��ȡ10.00mL��Һ���Ե�����ҺΪָʾ������Ũ��Ϊ0.500mol/LI2�ı���Һ���еζ�����Ӧԭ��Ϊ2S2O32-+I2=S4O62-+2I-��������ݼ�¼���±���ʾ��
��� | 1 | 2 | 3 |
��Һ�����/mL | 10.00 | 10.00 | 10.00 |
����I2����Һ�����/mL | 19.98 | 22.50 | 20.02 |
�ζ�ʱ���ﵽ�ζ��յ��������___________����Ʒ�Ĵ���Ϊ____________��
��6��Na2S2O3������������������Һ���ױ�Cl2������SO42-���÷�Ӧ�����ӷ���ʽΪ_________��
���𰸡� ������ƿ S2O32-+2H+=S��+SO2��+H2O 4SO2+2Na2S+Na2CO3=CO2+3Na2S2O3 Ϊ�˷�ֹ������©���д����������²��ʽ��� ����Ũ������ȴ�ᾧ ��Һ����ɫ����ɫ���Ұ���Ӳ���ɫ 82.67% S2O32-+4Cl2+5H2O=2SO42-+8Cl-+10H+
����������1������A������������ƿ����2�����������Ϣ����������ƣ�Na2S2O3��5H2O����������Һ�в����ȶ����ڣ�������Ӧ�����ӷ���ʽΪS2O32-+ 2H+ == S��+ SO2��+H2O������ʵ���в�������ҺpH <7����3�����������Ϣ������ƿ�м�����Na2S��Na2CO3��Һ��ͨ����SO2���Ʊ�Na2S2O3��Na2S ����Ԫ����-2��ʧ��������Na2S2O3��SO2��SԪ����+4�۵õ�������Na2S2O3�����ݻ��ϼ�������ȡ�ԭ���غ���ƽ���ܷ�Ӧ�Ļ�ѧ����ʽΪ4SO2+2Na2S+Na2CO3 =CO2+3Na2S2O3����4�������¶��ܽ���������Ϊ���ٲ�Ʒ����ʧ��������Ϊ���ȹ��˵�ԭ����Ϊ�˷�ֹ������©���д����������²��ʽ��ͣ��������ǵõ����壬���ʵ�����������Ũ������ȴ�ᾧ����5��������������ɫ����ﵽ�ζ��յ����������Һ����ɫ����ɫ���Ұ���Ӳ���ɫ�����ݱ������ݿ�֪�ڶ���ʵ�����̫����ȥ�����ı�Һ���ƽ��ֵ��20.00mL�����ĵ�����ʵ�����0.0500mol/L��0.02L=0.001mol��������������Ƶ����ʵ�����0.002mol��������0.002mol��248g/mol��0.486g�����Ʒ�Ĵ���Ϊ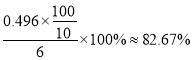 ����6��������Ŀ��Ϣ��֪��Na2S2O3����ˮ������Ӧ����Na2SO4��H2SO4����������ԭΪHCl����Ӧ�����ӷ���ʽΪS2O32��+ 4Cl2 + 5H2O��2SO42��+ 8Cl��+ 10H+ ��
����6��������Ŀ��Ϣ��֪��Na2S2O3����ˮ������Ӧ����Na2SO4��H2SO4����������ԭΪHCl����Ӧ�����ӷ���ʽΪS2O32��+ 4Cl2 + 5H2O��2SO42��+ 8Cl��+ 10H+ ��

����Ŀ����1L�����ܱ������г���X(g)��Y(g)��������ӦX(g)+Y(g)![]() M(g)+N(g)������ʵ���������±���
M(g)+N(g)������ʵ���������±���
ʵ���� | �¶�/�� | ��ʼʱ���ʵ���/mol | ƽ��ʱ���ʵ���/mol | |
n(X) | n(Y) | n(M) | ||
�� | 700 | 0.10 | 0.10 | 0.09 |
�� | 800 | 0.20 | 0.20 | 0.10 |
�� | 800 | 0.20 | 0. 30 | a |
�� | 900 | 0.10 | 0.15 | b |
����˵���������
A. ʵ����У���5minʱ���n(M) =0.05mol����0��5minʱ���ڣ���N��ʾ��ƽ����Ӧ����v(N) =0.01 mol/( L��min)
B. ʵ����У��÷�Ӧ��ƽ�ⳣ��K= 1.0
C. ʵ����У��ﵽƽ��ʱ��X��ת����Ϊ60%
D. ʵ����У��ﵽƽ��ʱ��b>0.06
����Ŀ������ʯ�����Ҫ�ɷ�ΪFe3O4��Al2O3��MnCO3��Mg0����MnO2�ȡ���ҵ�Ͻ�����ʯ����������������Ĥ��ⷨ���¼�����ȡ�����ڲ��Ƶ���ɫ��Ч��ˮ������(K2FeO4)����ҵ��������:

(1)��ϡ�����ȡ��ʯ�Ĺ����У�MnO2�ɽ�Fe2+����ΪFe3+��д���÷�Ӧ�����ӷ���ʽ:________��
(2)����Һ�е������ӳ�H+��Fe2+��Fe3+���_______(�����ӷ���)��
(3)��֪:��ͬ��������������������������������pH���±�:
���� | Fe3+ | A13+ | Fe2+ | Mn2+ | Mg2+ |
��ʼ������pH | 2.7 | 3.7 | 7.0 | 7.8 | 9.6 |
��ȫ������pH | 3.7 | 4.7 | 9.6 | 9.8 | 11.1 |
������е�����Һ��pH����6������pH���Լ����ѡ�����������Լ�:_______(��ѡ����ĸ����ͬ)����B�������ʺ�ɽ�һ����ȡK2FeO4����������B���������ѡ�����������Լ�:_____��
a.ϡ���� b.KOH c.��ˮ d.MnCO3 e.CaCO3
(4)����B����Ӧ�����ɸ�Чˮ�����������ӷ���ʽ_______________��
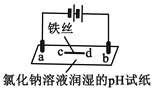
(5)���װ����ͼ��ʾ����ͷ��ʾ��Һ���������ƶ��ķ�������A�缫���ӵ���ֱ����Դ��_____�����������Һ��ϡ���ᣬ��������ֻ���̵���������������11g��ʱ����һ���缫�ϲ����������ڱ�״���µ����Ϊ________��

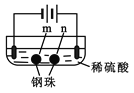
����Ŀ����ʯī�缫������е��ʵ�顣���ж�ʵ������Ľ��ͻ��Ʋⲻ��������
ʵ��һ | ʵ��� | |
װ�� |
|
|
���� | a��d����ֽ������b����죬�ֲ���ɫ��c�������Ա仯 | ����ʯī�缫���������ݲ�����n�������ݲ��������� |
A. a��d����2H2O+2e-=H2��+2OH-
B. b����2Cl--2e-=Cl2����Cl2����ˮ����HClO��ʹ��Һ��ɫ
C. c�������˷�Ӧ��Fe-2e-=Fe2+
D. ����ʵ��һ��ԭ����ʵ�����n��������O2