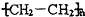��Ŀ����
9����1����֪��20��Cʱ��H2CO3��Ka1=4.2��10��7��Ka2=5.6��10��11��NH3•H2O��Kb=1.7��10��5��̼�������Һ��HCO3����NH4+��OH����H+��������Ũ���ɴ�С��˳��Ϊc��NH4+����c��HCO3-����c��OH-����c��H+����2���ǰ���NH2OH���ɿ����ǰ������ڵ�1����ԭ�ӱ��ǻ�ȡ�������ʣ���������ԭ�������Գ�ȥ��Fe2+�е�Fe3+������������һ�������ȶ�������Ⱦ�����壬д����Ӧ�����ӷ���ʽ2NH2OH+2Fe3+=2Fe2++N2��+2H2O+2H+��
���� ��1������ƽ�ⳣ��Խ�������̶�Խ����������ˮ��̶�ԽС��
��2���ǰ���NH2OH��������������һ�������ȶ�������Ⱦ�����壬���ǰ����������ɵ������ݴ�д����Ӧ�����ӷ���ʽ��
��� �⣺��1������ƽ�ⳣ��Խ�������̶�Խ����������ˮ��̶�ԽС�����ݵ���ƽ�ⳣ��֪������̶ȴ�С��ϵ��NH3•H2O��H2CO3��HCO3-����ˮ��̶�CO32-��HCO3-��NH4+������̼�������Һ�ʼ��ԣ���Һ�л�����̼������ӵȣ�����HCO-3��NH+4��OH-��H+��������Ũ���ɴ�С��˳��Ϊ��c��NH4+����c��HCO3-����c��OH-����c��H+����
�ʴ�Ϊ��c��NH4+����c��HCO3-����c��OH-����c��H+����
��2���ǰ���NH2OH���ɿ����ǰ������ڵ�1����ԭ�ӱ��ǻ�ȡ�������ʣ���������ԭ�������Գ�ȥ��Fe2+�е�Fe3+������������һ�������ȶ�������Ⱦ�����壬���ǰ����������ɵ�����ˮ���÷�Ӧ�����ӷ���ʽΪ��2NH2OH+2Fe3+=2Fe2++N2��+2H2O+2H+��
�ʴ�Ϊ��2NH2OH+2Fe3+=2Fe2++N2��+2H2O+2H+��
���� ���⿼��������Ũ�ȴ�С�Ƚϡ����ӷ���ʽ��д������ע�ظ�Ƶ����Ŀ��飬��Ŀ�Ѷ��еȣ�ע�������ε�ˮ��ԭ�������ӷ���ʽ��дԭ����ȷ�ж�����Ũ�ȴ�С���÷���������������ѧ�������Ӧ��������

 ���źþ���Ԫ����ĩ��ϵ�д�
���źþ���Ԫ����ĩ��ϵ�д�| A�� | ������ɱ�����в�������Ϊ���ɲ����ĵ��������ȱ��� | |
| B�� | ������Ư�۳���������ˮ�Ĵ��������ߵ�����ԭ����ͬ | |
| C�� | �Ȼ�����Һ����������ӡˢ��·������Ϊ����н�ǿ�����ԣ���ͭ�����û���Ӧ | |
| D�� | �������а뵼������ʣ����Կ����������ά |
| A�� | +$\frac{aM}{11.2m}$ | B�� | +$\frac{am}{11.2M}$ | C�� | +$\frac{11��2m}{aM}$ | D�� | +$\frac{aM}{22.4m}$ |
| A�� | ����HNO3�ữ��Ba��NO3��2��Һ | |
| B�� | �ȼ���HNO3�ữ���ټ�Ba��NO3��2 | |
| C�� | ���������ữ��BaCl2 | |
| D�� | ���������ữ�����г���������ˣ���Һ���ټ�BaCl2��Һ |
| A�� |  ��������ζ | B�� |  ȡ�ÿ�״���� | ||
| C�� |  �μ�Һ�� | D�� |  ϡ��Ũ���� |
| A�� | CH3CH2 CH2OH | B�� | CH3COOH | C�� | H2O | D�� | H2CO3 |
| A�� | Cl-��Cl2���ж� | B�� | Cl-��Cl2���ʻ���ɫ | ||
| C�� | ��Ƚϣ�Cl-�ȶ���Cl2���� | D�� | Cl-��Cl2������������ʷ�Ӧ |
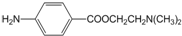 ���������ϳ�·����ͼ��ʾ�����ַ�Ӧ�Լ���������ʡ�ԣ���
���������ϳ�·����ͼ��ʾ�����ַ�Ӧ�Լ���������ʡ�ԣ���
 ��C�й����ŵ������ǰ������Ȼ���
��C�й����ŵ������ǰ������Ȼ��� ��1mol��������NaOH��Һ�����������2mol NaOH��
��1mol��������NaOH��Һ�����������2mol NaOH��