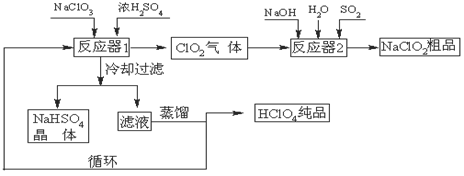
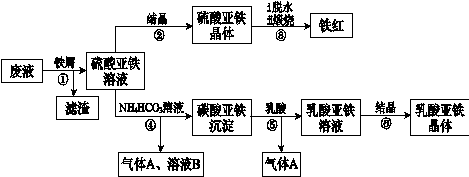
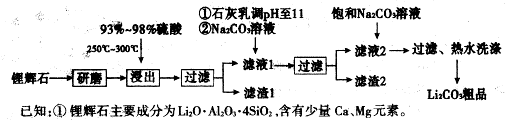
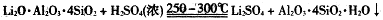��Ŀ����
����ɸ���о��ȵ��ṹ������ɸɸ�����ü���ͼ�����ڷ���ɸ�������������ߣ����ȶ���ǿ��������������û�е��ŵ㣬ʹ�÷���ɸ��ù㷺��Ӧ�á�ij���ͺŵķ���ɸ�Ĺ�ҵ�������̿ɼ�ʾ���£�

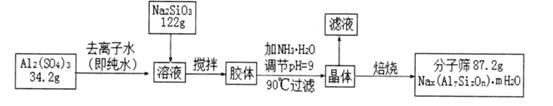
�ڼ�NH3��H2O����pH�Ĺ����У���pH���Ʋ�������Al(OH)3���ɣ�����������������Ԫ�غ�Ԫ�ؾ�û����ģ���ԭ�ӵ�������Ϊ10����
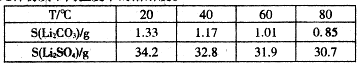
��1������ɸ�Ŀ�ֱ��Ϊ4A(1 A=10-10m)��Ϊ4A�ͷ���ɸ����Na+��Ca2+ȡ��ʱ���Ƶ�5A�ͷ���ɸ����Na+��K+ȡ��ʱ���Ƶ�3A�ͷ���ɸ��Ҫ��Ч����������(����ֱ��Ϊ4.65A)���춡��(����ֱ��Ϊ5.6A)Ӧ��ѡ�� �ͷ���ɸ��
��2��A12(SO4)3��Һ��Na2SiO3��Һ��Ӧ���ɽ�������ӷ���ʽΪ
��3��������������������Һ�ﺬ�е����ӳ�H+��OH-�⣬��ҪΪ ���������н��������ӵIJ���������
��4����NH3��H2O����pH���ȵ�90�沢���ȹ��˵�ԭ�������
��5�����������������÷���ɸ�Ļ�ѧʽΪ


�ڼ�NH3��H2O����pH�Ĺ����У���pH���Ʋ�������Al(OH)3���ɣ�����������������Ԫ�غ�Ԫ�ؾ�û����ģ���ԭ�ӵ�������Ϊ10����
��1������ɸ�Ŀ�ֱ��Ϊ4A(1 A=10-10m)��Ϊ4A�ͷ���ɸ����Na+��Ca2+ȡ��ʱ���Ƶ�5A�ͷ���ɸ����Na+��K+ȡ��ʱ���Ƶ�3A�ͷ���ɸ��Ҫ��Ч����������(����ֱ��Ϊ4.65A)���춡��(����ֱ��Ϊ5.6A)Ӧ��ѡ�� �ͷ���ɸ��
��2��A12(SO4)3��Һ��Na2SiO3��Һ��Ӧ���ɽ�������ӷ���ʽΪ
��3��������������������Һ�ﺬ�е����ӳ�H+��OH-�⣬��ҪΪ ���������н��������ӵIJ���������
��4����NH3��H2O����pH���ȵ�90�沢���ȹ��˵�ԭ�������
��5�����������������÷���ɸ�Ļ�ѧʽΪ
��12�֣�
��1��5A��1�֣�
��2�� 2A13++3SiO32-+ 6H2O=2Al(OH)3+3H2SiO3 (2��)
��3��Na+��NH4+��SO42-��2�֣� ����˿������˿�����ھƾ�����������������ɫʱ��պȡ������Һ�ٷ��ھƾ������������գ�������ʻ�ɫ��˵������Һ�к���Na+��2�֣�
��4�������ܴٽ��������ۣ����ȹ��˿ɷ�ֹ�������ʽᾧ���� ��2�֣�
��5��Na(AlSi3O12)��3H2O�� ( Na2O��Al2O3��10SiO2��6H2O)��3�֣����������𰸾����֣�
��1��5A��1�֣�
��2�� 2A13++3SiO32-+ 6H2O=2Al(OH)3+3H2SiO3 (2��)
��3��Na+��NH4+��SO42-��2�֣� ����˿������˿�����ھƾ�����������������ɫʱ��պȡ������Һ�ٷ��ھƾ������������գ�������ʻ�ɫ��˵������Һ�к���Na+��2�֣�
��4�������ܴٽ��������ۣ����ȹ��˿ɷ�ֹ�������ʽᾧ���� ��2�֣�
��5��Na(AlSi3O12)��3H2O�� ( Na2O��Al2O3��10SiO2��6H2O)��3�֣����������𰸾����֣�
����������ù��������������Ӻ�������ӷ���˫ˮ�ⷴӦ���õ����壬Ȼ��ͨ�����պ�õ�����ɸ��
��1������ֱ����С��4.65A��5.6A����ʹ��5A����ɸ���Է��룻
��2���÷�ӦΪ˫ˮ�ⷴӦ
��3�����ݼ�������ΪAl2(SO4)3��Na2SiO3��NH3��H2O����Al��Si��Naת���������У�����Һ����Na+��NH4+��SO42-������Na+����ɫ��Ӧ������NH4+��NaOH���ټ���NH3��
��5������ԭ�������ʼ��غ��ϵ����ȷ������ʽ��

��ϰ��ϵ�д�
�����Ŀ
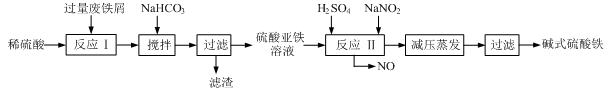
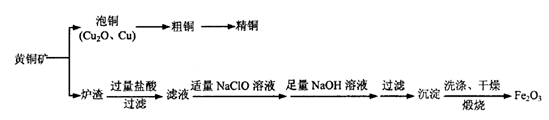
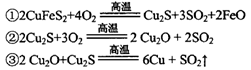
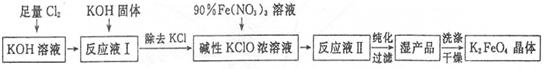
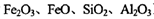 )���Ʊ�Fe2O3���������̻ش��������⣺
)���Ʊ�Fe2O3���������̻ش��������⣺