��Ŀ����
�������(K2Fe04)��һ�ּ�������������������һ������Ͷ��ˮ���������������������£�
��֪��2Fe(NO3)3+3KClO+10KOH=2K2FeO4+6KNO3+3KCl+5H2O
�ش�����������
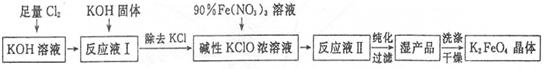
��1����Cl2ͨ��KOH��Һ�з�����Ӧ�����ӷ���ʽ��____________��
��2��д����ҵ����ȡCl2�Ļ�ѧ����ʽ____________��
��3���ڡ���ӦҺI���м���KOH�����Ŀ����____________��
��4��K2FeO4����Ϊ���Ͷ��ˮ��������ԭ����____________��
��5������KOH��Һʱ����61.6g KOH�����ܽ���100 mLˮ�У�������Һ���ܶ�Ϊ1.47 g ? mL-1�������Һ�����ʵ���Ũ��Ϊ____________��
��6���ӡ���ӦҺII���з����K2Fe04����Ʒ��___________ (д�� ѧ ʽ����
��7���ù���ÿ�õ�1.98kgK2FeO4������������Cl2�����ʵ���Ϊ______mol��

��֪��2Fe(NO3)3+3KClO+10KOH=2K2FeO4+6KNO3+3KCl+5H2O
�ش�����������
��1����Cl2ͨ��KOH��Һ�з�����Ӧ�����ӷ���ʽ��____________��
��2��д����ҵ����ȡCl2�Ļ�ѧ����ʽ____________��
��3���ڡ���ӦҺI���м���KOH�����Ŀ����____________��
��4��K2FeO4����Ϊ���Ͷ��ˮ��������ԭ����____________��
��5������KOH��Һʱ����61.6g KOH�����ܽ���100 mLˮ�У�������Һ���ܶ�Ϊ1.47 g ? mL-1�������Һ�����ʵ���Ũ��Ϊ____________��
��6���ӡ���ӦҺII���з����K2Fe04����Ʒ��___________ (д�� ѧ ʽ����
��7���ù���ÿ�õ�1.98kgK2FeO4������������Cl2�����ʵ���Ϊ______mol��
��1��Cl2+2OH- = Cl-+ ClO-+ H2O��2�֣�
��2��2NaCl + 2H2O 2NaOH + H2�� + Cl2����2�֣�
2NaOH + H2�� + Cl2����2�֣�
��3���롰��ӦҺ���й�����Cl2������Ӧ����KClO��2�֣�
��4��K2FeO4����ǿ�����ԣ���ɱ����������ԭ����FeԪ��Ϊ+3�ۣ���ˮ���γ�Fe(OH)3���壬������ˮ���������γɳ�������2�֣�
��5��10 mol��L-1��3�֣�
��6��KNO3 KCl��2�֣�
��7��15 ��2�֣�
��2��2NaCl + 2H2O
 2NaOH + H2�� + Cl2����2�֣�
2NaOH + H2�� + Cl2����2�֣���3���롰��ӦҺ���й�����Cl2������Ӧ����KClO��2�֣�
��4��K2FeO4����ǿ�����ԣ���ɱ����������ԭ����FeԪ��Ϊ+3�ۣ���ˮ���γ�Fe(OH)3���壬������ˮ���������γɳ�������2�֣�
��5��10 mol��L-1��3�֣�
��6��KNO3 KCl��2�֣�
��7��15 ��2�֣�
���������ע��տ�ʼͨ��������Cl2,�����Cl2�����K2FeO4�Ʊ�ʱ�ķ�Ӧ����K2FeO4�Ʊ����̿�֪����ƷΪKNO3��KCl�� (5)�и������ʵ���Ũ�ȶ�����㣬n(KOH)=1.1mol,V=
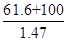 =109.9ml=0.11L�ɵ�C(KOH)= 10 mol��L-1����7������������ԭ�����غ㣬2Fe(NO3)3+3KClO+10KOH=2K2FeO4+6KNO3+3KCl+5H2O
=109.9ml=0.11L�ɵ�C(KOH)= 10 mol��L-1����7������������ԭ�����غ㣬2Fe(NO3)3+3KClO+10KOH=2K2FeO4+6KNO3+3KCl+5H2OCl2+2KOH=KCl+KClO+H2O, 2K2Fe04����3Cl2,
n(K2FeO4)=10mol,n(Cl2)=15mol

��ϰ��ϵ�д�
�����Ŀ

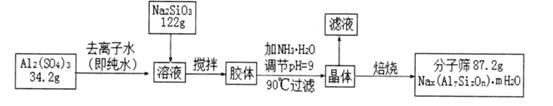
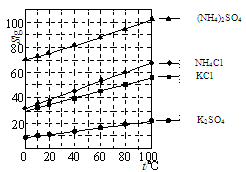


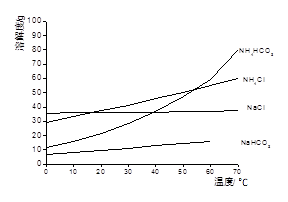
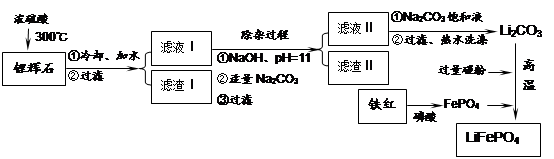
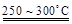 Li2SO4 + Al2O3��4SiO2��H2O��
Li2SO4 + Al2O3��4SiO2��H2O��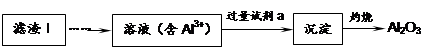
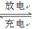 LiFePO4������еĹ������ʿɴ���Li������д���õ�طŵ�ʱ��������Ӧ�� �����øõ�ص�ⱥ��ʳ��ˮ�����ص缫��Ϊ���Ե缫������������������4480mL���壨��״��������ʱ���õ������﮵�����Ϊ ��
LiFePO4������еĹ������ʿɴ���Li������д���õ�طŵ�ʱ��������Ӧ�� �����øõ�ص�ⱥ��ʳ��ˮ�����ص缫��Ϊ���Ե缫������������������4480mL���壨��״��������ʱ���õ������﮵�����Ϊ ��