��Ŀ����
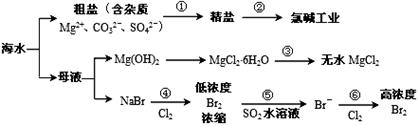
��ͼ�ǹ�ҵ������̼��﮵IJ��ֹ������̣����������ͼ����֪��Ϣ�ش����⡣
��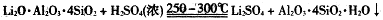
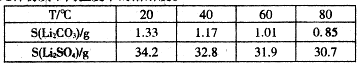
�ۼ������ʲ�ͬ�¶��µ��ܽ�ȡ�
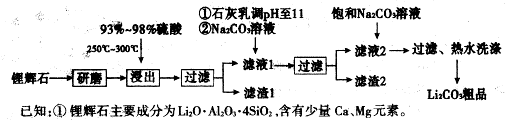
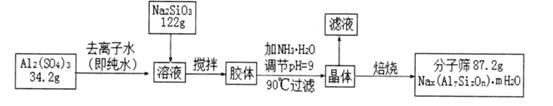
��1��������1�з����Al2O3�IJ�����������ͼ��ʾ�����ű�ʾ������Լ��������ʾ���õ������ʡ�д��ͼ�Т١��ڡ��۱�ʾ�ĸ����ʣ�����II�з�Ӧ�����ӷ���ʽ�� ��
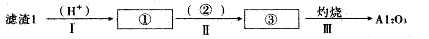
��2����֪����2����Ҫ�ɷ���Mg��OH��2��CaCO3��д����������2��Ӧ�����ӷ���ʽ��
��
��3������Һ2�м��뱥��Na2CO����Һ�����˺��á���ˮϴ�ӡ���ԭ���� ��
��4����ҵ�ϣ���Li2CO3��Ʒ�Ʊ��ɸߴ�Li2CO3�IJ��ֹ������¡�
�ٽ��ֲ�ƷLi2CO3�����������ý�۵�����Һ��LiOH��Һ������Һ������������ѡ���Ĥ�������ö��Ե缫��⡣�����ĵ缫��Ӧʽ�� ��
�ڵ������ƷLiOH��Һ�м������NH4HCO����Һ����Li2CO3��Ӧ�Ļ�ѧ����ʽ�� ��

��
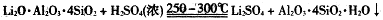
�ۼ������ʲ�ͬ�¶��µ��ܽ�ȡ�

��1��������1�з����Al2O3�IJ�����������ͼ��ʾ�����ű�ʾ������Լ��������ʾ���õ������ʡ�д��ͼ�Т١��ڡ��۱�ʾ�ĸ����ʣ�����II�з�Ӧ�����ӷ���ʽ�� ��
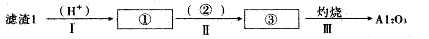
��2����֪����2����Ҫ�ɷ���Mg��OH��2��CaCO3��д����������2��Ӧ�����ӷ���ʽ��
��
��3������Һ2�м��뱥��Na2CO����Һ�����˺��á���ˮϴ�ӡ���ԭ���� ��
��4����ҵ�ϣ���Li2CO3��Ʒ�Ʊ��ɸߴ�Li2CO3�IJ��ֹ������¡�
�ٽ��ֲ�ƷLi2CO3�����������ý�۵�����Һ��LiOH��Һ������Һ������������ѡ���Ĥ�������ö��Ե缫��⡣�����ĵ缫��Ӧʽ�� ��
�ڵ������ƷLiOH��Һ�м������NH4HCO����Һ����Li2CO3��Ӧ�Ļ�ѧ����ʽ�� ��
��1�������Σ���Al3+���ڰ�ˮ������������������Һ��
Al3++3NH3��H2O=Al(OH)3��+3NH4+��Al3++3OH-=Al(OH)3��
��2��Mg2++2OH-=Mg(OH)2�� Ca2++CO32-=CaCO3��
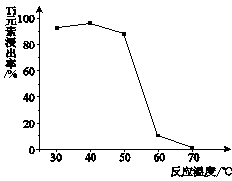
��3��Li2CO3�ܽ�����¶����߶���С����ˮϴ�ӿɼ���Li2CO3����ʧ��
��4����2Cl--2e-=Cl2
��2LiOH+NH4HCO3=Li2CO3+(NH4)2CO3+2H2O
Al3++3NH3��H2O=Al(OH)3��+3NH4+��Al3++3OH-=Al(OH)3��
��2��Mg2++2OH-=Mg(OH)2�� Ca2++CO32-=CaCO3��
��3��Li2CO3�ܽ�����¶����߶���С����ˮϴ�ӿɼ���Li2CO3����ʧ��
��4����2Cl--2e-=Cl2
��2LiOH+NH4HCO3=Li2CO3+(NH4)2CO3+2H2O
�����������1�����ݹ���ͼ�������յõ�Al2O3����ӦΪAl(OH)3,�����Һ��Ӧ�ú���Al3+��һ���Ʊ�Al(OH)3���ÿ����������백ˮ��Ӧ�����ԣ����ӷ���ʽΪAl3++3NH3��H2O=Al(OH)3��+3NH4+
��2����������2�������ڼ�����ʯ�����Na2CO3�������ӷ���ʽΪMg2++2OH-=Mg(OH)2�� Ca2++CO32-=CaCO3��
��3�������б����֪��Li2CO3�ܽ�����¶����߶���С����ˮϴ�ӿɼ���Li2CO3����ʧ��
��4���ٸ��������������ֲ�ƷLi2CO3�����������ý�۵�����Һ��LiOH��Һ������Һ������������ѡ���Ĥ�������ö��Ե缫��⡱����������������Ӧ��2Cl--2e-=Cl2
�ڸ��������֪��Ӧ����ʽ2LiOH+NH4HCO3=Li2CO3+(NH4)2CO3+2H2O

��ϰ��ϵ�д�
 ͬ��������ϰϵ�д�
ͬ��������ϰϵ�д� �ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�
�ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�
�����Ŀ


 Li7Ti5O12��3FePO4
Li7Ti5O12��3FePO4 


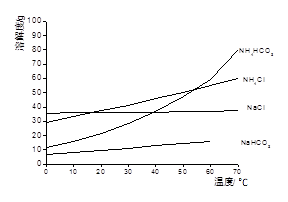
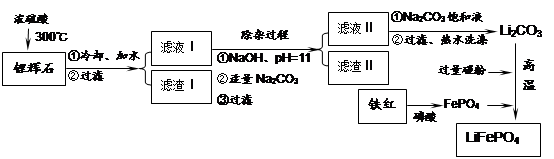
 Li2SO4 + Al2O3��4SiO2��H2O��
Li2SO4 + Al2O3��4SiO2��H2O��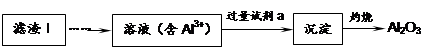
 LiFePO4������еĹ������ʿɴ���Li������д���õ�طŵ�ʱ��������Ӧ�� �����øõ�ص�ⱥ��ʳ��ˮ�����ص缫��Ϊ���Ե缫������������������4480mL���壨��״��������ʱ���õ������﮵�����Ϊ ��
LiFePO4������еĹ������ʿɴ���Li������д���õ�طŵ�ʱ��������Ӧ�� �����øõ�ص�ⱥ��ʳ��ˮ�����ص缫��Ϊ���Ե缫������������������4480mL���壨��״��������ʱ���õ������﮵�����Ϊ ��