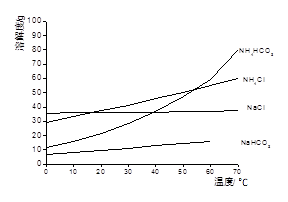��Ŀ����
��ҵ�����������ᣨ�е㣺90oC��ʱ��ͬʱ�������������ƣ��乤���������£�
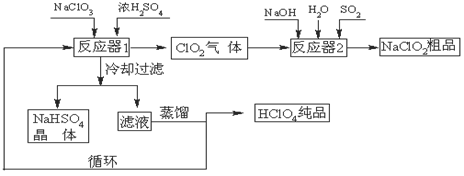
��1����ȴ���˵�Ŀ���ǽ���NaHSO4�� ���������NaHSO4���塣
��2����Ӧ��2�з�����Ӧ�����ӷ���ʽΪ ��SO2��
�������� ����
��3��������ҵ����������Ļ�ѧ��ӦΪ:3NaClO3+3H2SO4��Ũ����3NaHSO4+HClO4+2ClO2+H2O�����������뻹ԭ��������ʵ���֮��Ϊ ��
��4������ͨ��������Һ�ķ����õ��������ԭ������Ǹ�����ķе�Ƚ� ����ߡ��͡��������״���Һ���ݳ���ѭ��ʹ�õ������� ��

��1����ȴ���˵�Ŀ���ǽ���NaHSO4�� ���������NaHSO4���塣
��2����Ӧ��2�з�����Ӧ�����ӷ���ʽΪ ��SO2��
�������� ����
��3��������ҵ����������Ļ�ѧ��ӦΪ:3NaClO3+3H2SO4��Ũ����3NaHSO4+HClO4+2ClO2+H2O�����������뻹ԭ��������ʵ���֮��Ϊ ��
��4������ͨ��������Һ�ķ����õ��������ԭ������Ǹ�����ķе�Ƚ� ����ߡ��͡��������״���Һ���ݳ���ѭ��ʹ�õ������� ��
��1���ܽ�� ��2��2ClO2��SO2��4OH����2ClO2����SO42����2H2O�� ��ԭ
��3��1��2 ��4���� H2SO4
��3��1��2 ��4���� H2SO4
�����������1���ڷ�Ӧ��1�У������ƺ����ᷴӦ��õ��������Ƶ��ܽ�������¶ȵĽ��Ͷ���С��������ȴ���ˣ����Խ���NaHSO4���ܽ�Ȳ������NaHSO4���塣
��2���ڷ�Ӧ��2�У�����ʵ�ֶ���������NaClO2��ת�����������������Ϊ��ԭ����ClO2��ԭΪNaClO2����Ӧ�����ӷ���ʽΪ2ClO2��SO2��4OH����2ClO2����SO42����2H2O��
��3�����ݷ���ʽ3NaClO3+3H2SO4��Ũ����3NaHSO4+HClO4+2ClO2+H2O��֪��NaClO3������������Ҳ�ǻ�ԭ����������Ԫ�صĻ��ϼ۴ӣ�5�۲������ߵ���7�ۣ����ֽ��͵���4�ۣ����Ը�����������������������ǻ�ԭ������ݵ��ӵ�ʧ�غ��֪�����������뻹ԭ��������ʵ���֮��Ϊ1:2��
��4������ͨ��������Һ�ķ����õ������ᣬ��˵��������ķе�Ƚϵͣ��е㣺90oC��������ѭ��ͼ���Է���������Ϊ��Ӧ����뷴Ӧ��1�У�����Ϊ�������ڷ�Ӧ��2�����ɣ�����ѭ��ʹ�á�

��ϰ��ϵ�д�
�����Ŀ

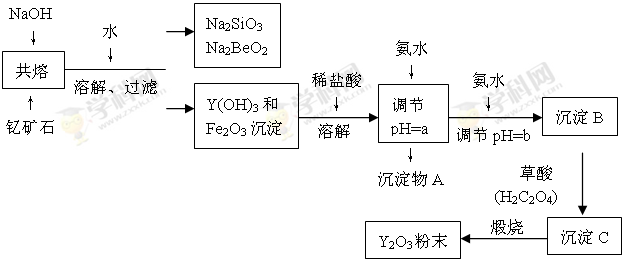
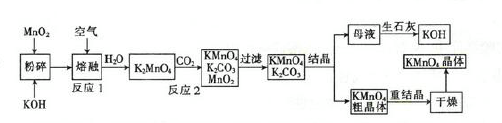

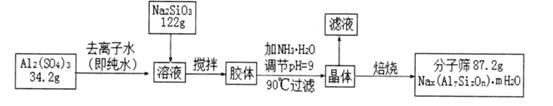

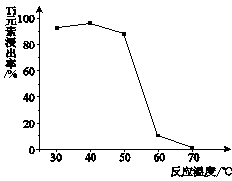
 Li7Ti5O12��3FePO4
Li7Ti5O12��3FePO4