��Ŀ����
��ҵ���û�ͭ��ұ��ͭ����¯���ۺ����õ�һ�ֹ����������£�
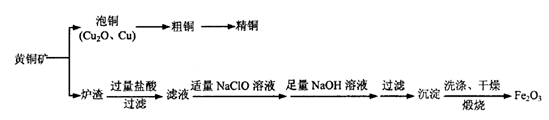
��1��ұ�������еõ�Cu2O��Cu�Ļ�����Ϊ����ͭ�����������A1�ڸ��������»�Ϸ�Ӧ�ɵô�ͭ����Ӧ��ѧ����ʽΪ________����ͭ����ʱӦ����ͭ������ֱ����Դ��____��������____���õ����Ƚϸߵľ�ͭ��
��2����ͳ��ͭ�ķ�����Ҫ�ǻ���ͭ������Ҫ��ӦΪ��
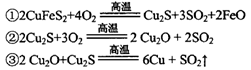
ÿ����1 mol Cu��������____mol O2����Ӧ���е���������____��
��3����ͭ������¯��(��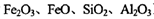 )���Ʊ�Fe2O3���������̻ش��������⣺
)���Ʊ�Fe2O3���������̻ش��������⣺
�ټ�������NaClO��Һ��Ŀ����_______ �������ӷ���ʽ��ʾ����
�ڳ�ȥAl3�������ӷ���ʽ��____��
��ѡ���ṩ���Լ������ʵ����֤¯���к���FeO���ṩ���Լ��У�ϡ���ᡢϡ���ᡢKSCN��Һ��KMnO4��Һ��NaOH��Һ����ˮ����ѡ�Լ���____��ʵ����ƣ�________��

��1��ұ�������еõ�Cu2O��Cu�Ļ�����Ϊ����ͭ�����������A1�ڸ��������»�Ϸ�Ӧ�ɵô�ͭ����Ӧ��ѧ����ʽΪ________����ͭ����ʱӦ����ͭ������ֱ����Դ��____��������____���õ����Ƚϸߵľ�ͭ��
��2����ͳ��ͭ�ķ�����Ҫ�ǻ���ͭ������Ҫ��ӦΪ��

ÿ����1 mol Cu��������____mol O2����Ӧ���е���������____��
��3����ͭ������¯��(��
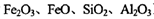 )���Ʊ�Fe2O3���������̻ش��������⣺
)���Ʊ�Fe2O3���������̻ش��������⣺�ټ�������NaClO��Һ��Ŀ����_______ �������ӷ���ʽ��ʾ����
�ڳ�ȥAl3�������ӷ���ʽ��____��
��ѡ���ṩ���Լ������ʵ����֤¯���к���FeO���ṩ���Լ��У�ϡ���ᡢϡ���ᡢKSCN��Һ��KMnO4��Һ��NaOH��Һ����ˮ����ѡ�Լ���____��ʵ����ƣ�________��
��1��3Cu2O+2Al
 Al2O3+6Cu (2��) �� (1��) �� (1��)
Al2O3+6Cu (2��) �� (1��) �� (1��)��2��2.5 (1��) Cu2O��Cu2S (1��)
��3����2Fe2++ClO-+2H+=2Fe3++Cl-+H2O (2��)
��Al3++4OH-=AlO2-+2H2O (2��)
��ϡ���� ���������Һ (2��)
ȡ�������������Һ���Թ��У��μӾ�ϡ�����ܽ��¯����Һ�����������Һ����ɫ��ȥ��֤������Fe2+ (2��)
�����������1���������֪������ͭ�������A1�ڸ��������»�Ϸ�Ӧ�ɵô�ͭΪ���ȷ�Ӧ����⾫��ͭ�Ǵ�ͭ�������������ӵ�Դ��������2������Ŀ����������ʽ������ɵã�2CuFeS2+5O2=2FeO+4SO2+2Cu��ÿ����1 mol Cu��������2.5mol O2������ͭ�Ļ��ϼ۽��ͣ���Cu2O��Cu2S����������3��¯���к���FeO����Ҫ�����������������л�ԭ�ԣ��ʼ���ʱ��ѡ�����Ը�����ء�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ